Normal cells are the building blocks of all living organisms, performing specific functions vital for the body’s overall health and homeostasis.
Each normal cell type has a distinct role, such as muscle cells contracting to facilitate movement, red blood cells transporting oxygen, and nerve cells transmitting signals.
Structurally, normal cells have a well-organized nucleus containing DNA, mitochondria for energy production, and various other organelles that support their functions. They follow a regulated cell cycle, growing, dividing, and dying in a controlled manner to maintain tissue health.
Cancer Cell Characteristics: Cancer cells, on the other hand, are characterized by their uncontrolled growth and division. Unlike normal cells, cancer cells do not respond to the body’s regulatory signals.
Cancer cells can evade apoptosis, the programmed cell death that typically eliminates damaged or unnecessary cells, allowing them to persist and proliferate abnormally.
How Does a Normal Cell Become a Cancer Cell
A normal cell becomes a cancer cell through genetic mutations that disrupt the regulation of cell growth, division, and death. These mutations can be caused by factors like carcinogens, viruses, or inherited genetic predispositions.
Key changes include the activation of oncogenes (genes that promote uncontrolled cell division) and the inactivation of tumor suppressor genes (which normally prevent excessive growth).
Cancer Cells vs Normal Cells Under Microscope
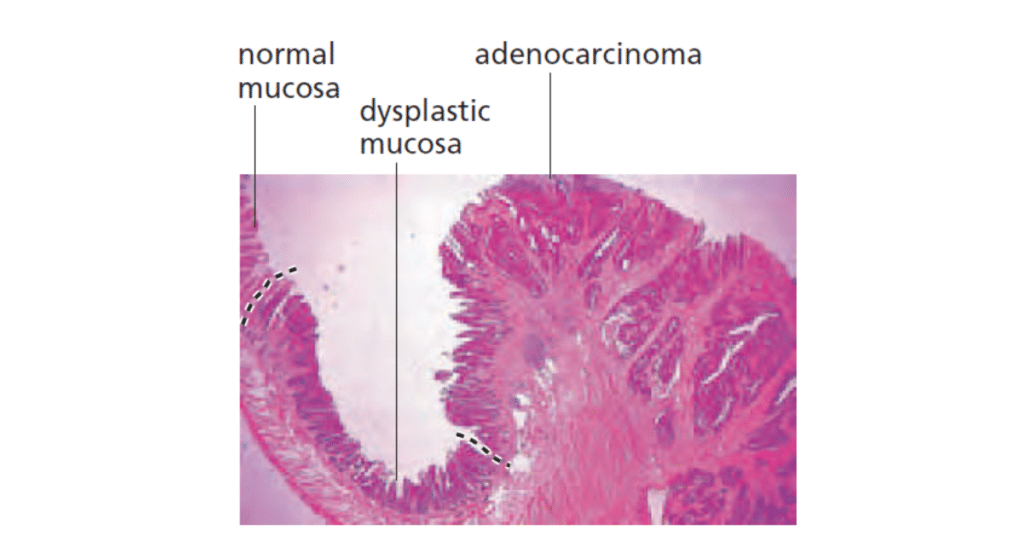
Under a microscope, cancer cells and normal cells exhibit several distinct differences:
Normal Cells:
- Shape: Normal cells have a uniform, regular shape, often round or oval.
- Size: They are relatively uniform in size.
- Nucleus: The nucleus is round, centrally located, and contains evenly distributed chromatin.
- Cell Division: Normal cells divide in a controlled manner with distinct phases of the cell cycle.
- Arrangement: They typically form organized tissue structures and have contact inhibition (stopping growth when they touch other cells).
Cancer Cells:
- Shape: Cancer cells are often irregular in shape, with uneven borders.
- Size: They vary in size, often becoming larger or smaller than normal cells.
- Nucleus: The nucleus is often enlarged, with an irregular shape and abnormal chromatin distribution (more dense or clumped).
- Cell Division: Cancer cells divide uncontrollably, and may have an abnormal number of chromosomes (aneuploidy).
- Arrangement: They grow in a disorganized manner, often forming tumors. Cancer cells may lose contact inhibition, leading to abnormal growth and spread.
These differences reflect the underlying uncontrolled growth and abnormal behavior of cancer cells compared to normal, healthy cells.
Overview of Cell Cycle and Division
Normal Cell Cycle Regulation: The cell cycle is a series of phases that normal cells go through to grow and divide. It includes the G1 phase (cell growth), S phase (DNA replication), G2 phase (preparation for division), and M phase (mitosis, where the cell divides into two daughter cells).
This process is tightly regulated by checkpoints and various proteins, such as cyclins and cyclin-dependent kinases (CDKs), ensuring that cells only divide when they are healthy and conditions are favorable.
Abnormal Cell Division in Cancer: In cancer cells, the regulation of the cell cycle is disrupted. Mutations in genes that control the cell cycle checkpoints can lead to uncontrolled cell division.
For example, mutations in the TP53 gene, which encodes the p53 protein responsible for DNA damage repair and cell cycle arrest, can allow cells with damaged DNA to continue dividing. This unregulated proliferation contributes to tumor growth and the progression of cancer.
Difference Between Normal Cells and Cancer Cells Table
Here is a table summarizing the key differences between cancer cells vs normal cells:
| Feature | Normal Cells | Cancer Cells |
|---|---|---|
| Cell Proliferation | Controlled, regulated by the cell cycle | Uncontrolled, rapid, and often continuous |
| Apoptosis | Undergo programmed cell death when damaged | Evade apoptosis, leading to survival of abnormal cells |
| Genetic Stability | Maintain genetic stability | High genetic instability with frequent mutations |
| Energy Production | Primarily oxidative phosphorylation | Predominantly glycolysis (Warburg effect) |
| Nutrient Utilization | Regulated, balanced nutrient uptake | Increased nutrient uptake to support rapid growth |
| Growth Factors | Require external growth signals to divide | Often produce their own growth signals (autocrine signaling) |
| Cellular Communication | Normal cell signaling pathways | Disrupted signaling, often leading to growth advantage |
| Response to Anti-growth Signals | Responsive to anti-growth signals | Often ignore anti-growth signals |
| Cell Structure | Well-differentiated, organized cytoskeleton | Poorly differentiated, disorganized cytoskeleton |
| Angiogenesis | Controlled blood vessel formation | Promotes excessive angiogenesis to support tumor growth |
| Metastasis | Do not metastasize | Capable of metastasis, spreading to other body parts |
| Cell Cycle Checkpoints | Functional, prevent damaged cells from dividing | Often dysfunctional, allowing division of damaged cells |
| Contact Inhibition | Exhibit contact inhibition, stop growing when touching other cells | Lack contact inhibition, continue to grow even when in contact with other cells |
| Tumor Microenvironment | Normal interaction with surrounding cells | Alter and manipulate the tumor microenvironment to support cancer growth |
This table highlights the fundamental differences between cancer cells vs normal cells, illustrating how cancer cells adopt various mechanisms to sustain uncontrolled growth and evade normal regulatory processes.
Genetic and Molecular Differences Between Cancer Cells vs Normal Cells
DNA and Chromosomal Changes
Role of Genetic Mutations in Cancer Cells: Genetic mutations are a hallmark of cancer cells and play a critical role in their development and progression. These mutations can occur in various forms, such as point mutations, insertions, deletions, and chromosomal rearrangements.
They can affect genes that control cell growth, division, and death, leading to uncontrolled cell proliferation. Common mutations in cancer include those in oncogenes (e.g., KRAS, BRAF) and tumor suppressor genes (e.g., TP53, RB1).
These genetic alterations can drive the transformation of normal cells into cancerous ones by disrupting normal cellular processes.
Chromosomal Abnormalities in Cancer: Cancer cells often exhibit significant chromosomal abnormalities, including aneuploidy (abnormal number of chromosomes), translocations, and amplifications.
These changes can lead to the loss of tumor suppressor genes or the overexpression of oncogenes, further promoting cancer development.
For example, the Philadelphia chromosome, a result of a translocation between chromosomes 9 and 22, is associated with chronic myeloid leukemia (CML).
Oncogenes and Tumor Suppressor Genes
Function of Oncogenes in Normal Cells: Oncogenes are genes that normally help cells grow. In normal cells, these genes are tightly regulated and play a role in normal cell growth and division.
However, when these genes are mutated or overexpressed, they become oncogenes that can drive cancer development.
For example, the RAS gene family includes oncogenes that, when mutated, can lead to uncontrolled cell growth and cancer.
Impact of Tumor Suppressor Gene Loss in Cancer: Tumor suppressor genes are crucial for regulating cell division and preventing tumor formation. They act as the “brakes” of the cell cycle, ensuring that cells do not grow and divide uncontrollably.
Mutations or deletions in these genes can lead to the loss of their function, contributing to cancer development.
The TP53 gene, is one of the most well-known tumor suppressor genes. Mutations in TP53 are found in more than 50% of human cancers, underscoring its critical role in maintaining normal cell function and preventing cancer.
Cell Signaling Pathways of Cancer Cells vs Normal Cells
Cell signaling pathways are essential for normal cell communication and function. These pathways involve a series of molecular interactions that transmit signals from the cell surface to the nucleus, regulating various cellular activities such as growth, differentiation, and apoptosis.
Key signaling pathways in normal cells include the PI3K/AKT pathway, the MAPK/ERK pathway, and the Wnt signaling pathway. These pathways ensure that cells respond appropriately to external signals and maintain homeostasis.
Disrupted Signaling Pathways in Cancer Cells: In cancer cells, normal cell signaling mechanisms are often disrupted, leading to uncontrolled growth and survival. Mutations in genes encoding signaling proteins can result in constitutive activation or inhibition of these pathways.
For example, mutations in the PI3K/AKT pathway can lead to enhanced cell survival and proliferation, contributing to cancer progression. Similarly, aberrant activation of the Wnt signaling pathway is implicated in various cancers, including colorectal cancer.
Cellular Behavior and Growth Patterns of Cancer Cells vs Normal Cells
Cell Proliferation and Apoptosis
Regulation of Cell Proliferation in Normal Cells: In normal cells, proliferation is a tightly controlled process governed by the cell cycle. Cells only divide in response to specific signals and conditions, such as growth factors and nutrients.
Evasion of Apoptosis by Cancer Cells: Apoptosis, or programmed cell death, is a crucial mechanism that allows the body to eliminate damaged or unnecessary cells. Normal cells undergo apoptosis in response to signals indicating DNA damage, infection, or other forms of cellular stress.
In contrast, cancer cells often develop mechanisms to evade apoptosis, allowing them to survive and proliferate despite being damaged or abnormal. This evasion can result from mutations in genes regulating apoptosis, such as TP53, or overexpression of anti-apoptotic proteins like BCL-2.
Metastasis and Tumor Formation
How Cancer Cells Spread (Metastasis): Metastasis is the process by which cancer cells spread from the primary tumor to distant organs and tissues.
This involves several steps, including detachment from the primary tumor, invasion into surrounding tissues, entry into the bloodstream or lymphatic system, and colonization of new sites.
Cancer cells must acquire specific traits to metastasize, such as increased motility, epithelial to mesenchymal transition, the ability to degrade extracellular matrix components, and resistance to anoikis (cell death induced by detachment).
Metastasis is a major cause of cancer-related mortality, making it a critical area of research for developing interventions that can prevent or limit the spread of cancer.
Differences in Tumor Microenvironment: The tumor microenvironment (TME) refers to the environment surrounding a tumor, including various cell types, extracellular matrix components, and signaling molecules.
The TME plays a significant role in cancer progression and metastasis. In normal tissues, cells exist in a balanced microenvironment that supports their function and inhibits uncontrolled growth.
In contrast, the TME in cancer is often characterized by hypoxia (low oxygen levels), acidic conditions, and the presence of immunosuppressive cells. These factors can promote cancer cell survival, proliferation, and invasion.
Angiogenesis
Angiogenesis is the process by which new blood vessels form from pre-existing vessels. In normal tissues, angiogenesis is a tightly regulated process that occurs during wound healing, embryonic development, and the menstrual cycle.
It ensures that tissues receive an adequate supply of oxygen and nutrients. Key regulators of angiogenesis include vascular endothelial growth factor (VEGF) and angiopoietins.
Cancer cells can hijack the angiogenic process to support their growth and survival. They often secrete high levels of pro-angiogenic factors like VEGF, stimulating the formation of new blood vessels to supply the tumor with oxygen and nutrients.
This process, known as tumor angiogenesis, is critical for tumor growth and metastasis. The newly formed blood vessels in tumors are typically abnormal and leaky, contributing to a chaotic and dysfunctional tumor microenvironment.
Metabolic Differences Between Cancer Cells vs Normal Cells
Energy Production in Cells
Cellular Metabolism in Normal Cells: In normal cells, energy production primarily occurs through oxidative phosphorylation within the mitochondria. This process involves the breakdown of glucose into pyruvate via glycolysis in the cytoplasm, followed by the citric acid cycle and electron transport chain in the mitochondria.
The end result is the production of adenosine triphosphate (ATP), the cell’s main energy currency, alongside water and carbon dioxide. This efficient energy production process ensures that normal cells have adequate ATP to perform various functions, including growth, repair, and maintenance of cellular homeostasis.
Altered Metabolism in Cancer Cells (Warburg Effect): Cancer cells often exhibit a phenomenon known as the Warburg effect, where they predominantly rely on glycolysis for energy production, even in the presence of sufficient oxygen.
This metabolic shift results in the increased conversion of glucose to lactate, producing less ATP compared to oxidative phosphorylation but at a faster rate. This adaptation allows cancer cells to generate the necessary energy and metabolic intermediates required for rapid proliferation and growth.
The Warburg effect also contributes to the acidic microenvironment of tumors, promoting further cancer cell invasion and metastasis.
Nutrient Utilization
How Normal Cells Utilize Nutrients: Normal cells obtain nutrients from the bloodstream, including glucose, amino acids, fatty acids, and vitamins. These nutrients are utilized for energy production, synthesis of macromolecules (proteins, lipids, nucleic acids), and various cellular functions.
Nutrient uptake in normal cells is tightly regulated to ensure a balance between supply and demand, maintaining cellular and tissue homeostasis.
Changes in Nutrient Uptake by Cancer Cells Cancer cells often exhibit increased nutrient uptake to support their rapid growth and division. They upregulate glucose and amino acid transporters on their cell membranes, allowing greater influx of these essential nutrients.
Additionally, cancer cells can alter their lipid metabolism to provide necessary components for membrane synthesis and energy storage. These metabolic adaptations enable cancer cells to sustain their heightened proliferative state and survive in nutrient-deprived environments.
Implications for Cancer Treatment and Research
Targeted Therapies
Development of Targeted Cancer Therapies The understanding of genetic and molecular differences between cancer cells and normal cells has revolutionized cancer treatment through the development of targeted therapies.
These treatments are designed to specifically target the genetic mutations and molecular pathways that drive cancer cell growth and survival. Examples include tyrosine kinase inhibitors (TKIs) like imatinib, which targets the BCR-ABL fusion protein in chronic myeloid leukemia (CML), and monoclonal antibodies like trastuzumab, which targets HER2-positive breast cancer.
Immunotherapy Advances
Role of Immunotherapy in Cancer Treatment Immunotherapy harnesses the body’s immune system to recognize and attack cancer cells. Recent advances in immunotherapy have shown significant promise in treating various types of cancer.
Checkpoint inhibitors, such as pembrolizumab and nivolumab, block proteins like PD-1 and CTLA-4 that inhibit immune responses, thereby enhancing the ability of immune cells to attack cancer.
CAR-T cell therapy, another form of immunotherapy, involves modifying a patient’s T cells to express chimeric antigen receptors (CARs) that specifically target cancer cells.
Personalized Medicine
Importance of Personalized Medicine in Cancer Treatment Personalized medicine, or precision medicine, tailors treatment to the individual characteristics of each patient’s cancer.
This approach relies on the detailed genetic and molecular profiling of tumors to identify specific mutations and biomarkers that can guide treatment decisions.
By selecting therapies that are most likely to be effective based on the unique genetic makeup of a patient’s cancer, personalized medicine aims to improve outcomes and reduce unnecessary side effects.
Technologies Enabling Personalized Medicine Advances in genomic sequencing, bioinformatics, and molecular diagnostics have been critical in enabling personalized medicine.
Next-generation sequencing (NGS) allows for the comprehensive analysis of cancer genomes to identify actionable mutations and potential therapeutic targets.
Liquid biopsies, which analyze circulating tumor DNA (ctDNA) in the blood, offer a non-invasive method to monitor tumor genetics and response to treatment over time. These technologies are transforming cancer care by providing more precise and dynamic approaches to treatment.
Ongoing Research and Future Perspectives
Current Trends in Cancer Research: Cancer research is continually evolving, with ongoing efforts to better understand the underlying biology of cancer and develop more effective treatments.
Current trends include the study of cancer stem cells, which are believed to be responsible for tumor initiation and resistance to treatment, and the exploration of the tumor microenvironment, including its role in cancer progression and response to therapy.
Advances in artificial intelligence and machine learning are also being applied to cancer research, aiding in the analysis of complex data sets and the identification of new therapeutic targets.
Future Directions in Cancer Treatment The future of cancer treatment lies in the integration of multiple therapeutic approaches to achieve better outcomes. Combining targeted therapies, immunotherapies, and traditional treatments like chemotherapy and radiation may provide synergistic effects and overcome resistance.
Additionally, the development of novel treatments, such as oncolytic viruses and cancer vaccines, holds promise for the future. Continued investment in cancer research, coupled with advancements in technology will drive the discovery of new treatments and improve the prognosis for cancer patients.
Conclusion
In conclusion, the comparison of cancer cells vs normal cells reveals significant differences, including the uncontrolled growth, irregular shape, and abnormal nuclei of cancer cells, while normal cells maintain an organized and regulated structure. These distinctions are key to understanding how cancer cells behave and contribute to tumor progression.


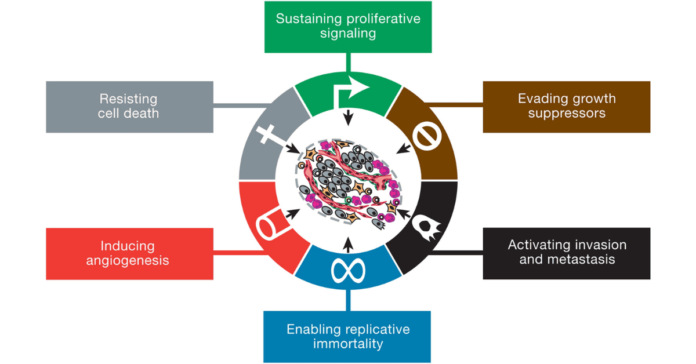
[…] Read more […]