Welcome to the world of protein analysis, where understanding how to read a Western blot effectively is a crucial skill for every scientist and researcher. Western blotting is a powerful laboratory technique that allows us to interpret protein levels and gain insights into various cellular processes.
Interpreting protein levels accurately is essential for uncovering the molecular mechanisms underlying biological phenomena, such as disease progression or response to treatment. By mastering the art of Western blot analysis, you will be equipped with a versatile tool to explore protein expression patterns, detect specific proteins, and deepen your understanding of cellular events at the molecular level.
Throughout this comprehensive guide, we will take you on a journey through the step-by-step process of Western blotting. From sample preparation to protein detection and data analysis, we will cover all the essentials needed to read a Western blot like a pro. By the end of this article, you will have the knowledge and confidence to generate reliable and meaningful results for your research.
Key Takeaways:
- Understanding how to read a Western blot is essential for interpreting protein levels accurately and studying cellular events at the molecular level.
- Western blotting is a versatile laboratory technique that allows for the detection of specific proteins and analysis of protein expression patterns.
- Mastering the step-by-step process of Western blotting, from sample preparation to data analysis, is crucial for generating reliable and meaningful results.
- Interpreting Western blot results requires skills in protein detection and quantification, as well as troubleshooting common pitfalls.
- As the field of protein analysis advances, future developments in Western blotting techniques offer exciting possibilities for multiplexing, quantitative analysis, and novel detection methods.
What is a Western Blot?
A Western blot is a widely used laboratory technique for protein analysis. It plays a crucial role in identifying and quantifying specific proteins within a sample. By separating proteins based on their molecular weight, a Western blot enables researchers to detect and analyze target proteins with high precision.
The Western blotting process involves several key steps:
- Sample Preparation: The protein of interest is extracted and denatured, preparing it for separation.
- Gel Electrophoresis: The proteins are loaded onto a gel and separated based on their size using an electrical current.
- Transfer onto a Membrane: The separated proteins are transferred to a membrane, allowing for downstream detection.
- Blocking: The membrane is treated with blocking agents to prevent non-specific binding of antibodies.
- Antibody Incubation: Primary and secondary antibodies are applied to the membrane to specifically bind to the target protein.
- Protein Detection: The target protein is visualized using detection methods such as chemiluminescence or fluorescence.
- Result Visualization and Analysis: The Western blot results are captured, quantified, and analyzed to extract meaningful data.
Through this process, a Western blot provides valuable insights into protein expression levels and molecular weights, allowing researchers to investigate biological processes and diseases.
| Advantages of Western Blotting | Limitations of Western Blotting |
|---|---|
| Accurate detection and quantification of target proteins. Ability to analyze multiple proteins simultaneously. Reliable detection of low-abundance proteins. | Time-consuming process. Requires specialized equipment and expertise. Possible variations in results due to experimental conditions. |
Sample Preparation for Western Blotting
Sample preparation is a crucial step in Western blotting, as it directly affects the quality and reliability of the results. Proper sample preparation ensures efficient protein extraction, denaturation, and loading onto the gel for optimal separation and detection.
Protein Extraction
Effective protein extraction is the foundation of a successful Western blot. Various methods can be used to extract proteins from different sample types, such as cells, tissues, or biofluids. Some common techniques include:
- RIPA Lysis Buffer: A widely used buffer for general protein extraction, containing detergents and protease inhibitors.
- Urea or Guanidine Hydrochloride: These strong denaturants can solubilize proteins for extracting difficult-to-solubilize samples.
- Phosphoprotein Extraction Buffer: Specifically designed for extracting phosphorylated proteins, preserving their phosphorylation states.
It is essential to optimize the extraction method based on the sample type and the proteins of interest to obtain high-quality protein lysates.
Denaturation
Denaturation is a critical step in Western blotting that ensures protein unfolding, facilitating their separation based on molecular weight. Denaturing agents, such as heat or chemical denaturants, are used to disrupt protein structures and expose linear epitopes for antibody binding. Common denaturation methods include:
- Boiling: Heat denaturation by boiling the lysate sample effectively denatures proteins.
- Chemical Denaturants: Chemical agents like urea or guanidine hydrochloride can be used for denaturation.
Loading onto a Gel
Once proteins are extracted and denatured, they need to be loaded onto a polyacrylamide gel for separation. The loading process ensures that proteins are evenly distributed for accurate analysis. Key considerations for loading include:
- Sample Volume: The amount of protein loaded must be optimized to avoid overloading or insufficient detection.
- Protein Markers: Including protein standards alongside the samples helps determine the molecular weight of the proteins.
The image above visually represents the process of protein extraction, highlighting the critical steps involved in sample preparation for Western blotting.
Gel Electrophoresis and Protein Separation
In Western blotting, one of the critical steps for accurate protein analysis is gel electrophoresis. Gel electrophoresis allows for the separation of proteins based on their molecular weight, facilitating the identification and quantification of specific protein bands.
During gel electrophoresis, the protein samples are loaded onto a polyacrylamide gel and subjected to an electric field. Proteins, being negatively charged, migrate through the gel towards the positive electrode, with smaller proteins moving faster than larger ones.
The separation of proteins by size enables the visualization of distinct bands on the gel, which correspond to different proteins present in the sample. These bands form the basis for further analysis and detection in the Western blotting process.
To further enhance the separation of proteins, a technique called SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis) is commonly employed. SDS-PAGE denatures proteins and coats them with a negatively charged SDS detergent, ensuring consistent migration solely based on their molecular weight.
To visualize the separated protein bands, staining techniques such as Coomassie Brilliant Blue or silver stain are commonly used. These staining methods allow for the detection of protein bands that can be analyzed and quantified for subsequent interpretation.
Advantages of gel electrophoresis in protein separation:
- Enables separation of proteins based on molecular weight
- Provides a foundation for further analysis and detection
- Allows for quantification and comparison of protein levels
Comparison of different gel electrophoresis techniques
| Gel Electrophoresis Technique | Advantages | Disadvantages |
|---|---|---|
| Agarose gel electrophoresis | Simple and cost-effective | Limited resolution for protein separation |
| Polyacrylamide gel electrophoresis (PAGE) | High resolution for protein separation | Time-consuming and requires specialized equipment |
| Two-dimensional gel electrophoresis (2D-PAGE) | Allows for separation of complex protein mixtures | Complex and requires advanced data analysis |
In summary, gel electrophoresis plays a crucial role in protein separation in Western blotting. By exploiting the differences in molecular weight, this technique enables the visualization and analysis of distinct protein bands, laying the foundation for accurate detection and interpretation of Western blot results.
Transfer of Proteins onto a Membrane
After the completion of gel electrophoresis and protein separation, the next crucial step in Western blotting is the transfer of proteins from the gel onto a membrane. This step is essential for further analysis and detection of specific proteins of interest.
There are two commonly used methods for protein transfer: wet transfer and semi-dry transfer. Wet transfer involves assembling a transfer sandwich with the gel, membrane, and filter paper soaked in transfer buffer. The transfer occurs through electrophoretic principles with the help of an electric field. This method is ideal for transferring proteins of varying sizes and is known for its efficiency.
Semi-dry transfer, on the other hand, utilizes a specialized apparatus that applies both current and voltage across the sandwiched gel and membrane. This technique provides faster transfer times and requires less buffer volume. It is suitable for transferring smaller proteins where speed is of the essence.
It is important to note that the success of protein transfer depends on the appropriate transfer conditions. Factors such as transfer time, current, voltage, and buffer pH can significantly impact the efficiency and quality of transfer. Optimization of these parameters is necessary to ensure accurate and reliable results.
| Transfer Method | Advantages | Disadvantages |
|---|---|---|
| Wet Transfer | Efficient transfer for proteins of varying sizes Compatibility with different membrane types Higher protein retention on the membrane | Longer transfer times Requirement of larger buffer volumes |
| Semi-Dry Transfer | Faster transfer times Lower buffer requirement Suitability for smaller proteins | Less protein retention on the membrane Limited compatibility with certain membrane types |
Blocking and Antibody Incubation
In Western blotting, the blocking step plays a crucial role in preventing nonspecific binding and reducing background noise. It involves saturating unoccupied binding sites on the membrane to minimize nonspecific interactions between the antibody and the membrane. Proper blocking enhances the efficiency and specificity of antibody detection, improving the overall accuracy of protein detection.
Common blocking agents employed in Western blotting:
- Nonfat milk: A widely used blocking agent that provides effective blocking while being cost-effective.
- Bovine serum albumin (BSA): Another popular blocking agent that offers excellent protein stabilization and reduces non-specific binding.
- Casein: This protein-based blocking agent provides efficient blocking and has minimal interference with antibody binding.
The specific requirements of the experiment and the type of antibody being used determine the choice of the right blocking agent.
Once the blocking step is complete, the next critical stage is antibody incubation. This step involves adding the primary antibody, which specifically recognizes and binds to the target protein of interest. The antibody is incubated with the membrane, allowing for the formation of antigen-antibody complexes. The primary antibody binds to the target protein, enabling its detection.
The primary antibody used in Western blotting may be polyclonal or monoclonal, with each having its own advantages. Polyclonal antibodies are produced by injecting an animal with the target antigen, resulting in a heterogeneous mixture of antibodies that recognize different epitopes on the target protein. Monoclonal antibodies, on the other hand, are produced by fusing immortalized B cells with antibody-producing cells, resulting in a more homogeneous population of antibodies that recognize a single epitope.
Table: Comparison of Blocking Agents
| Blocking Agent | Advantages | Disadvantages |
|---|---|---|
| Nonfat Milk | Cost-effective | May interfere with antibody binding at high concentrations |
| Bovine Serum Albumin (BSA) | Excellent protein stabilization | Can lead to increased background signal at high concentrations |
| Casein | Efficient blocking | Higher cost compared to other blocking agents |
Protein Detection Methods
In Western blotting, selecting the appropriate protein detection method is crucial for accurately analyzing protein samples. This section explores two commonly used detection methods: chemiluminescence and fluorescence. Understanding the advantages and limitations of each technique will help researchers choose the most suitable approach for their experiments.
Chemiluminescence
Chemiluminescence is a widely utilized protein detection method in Western blotting. It involves the use of enzyme-linked antibodies that produce light as a result of a chemical reaction. This emitted light can then be detected using specialized imaging systems or X-ray films. One of the key advantages of chemiluminescence is its high sensitivity, allowing for the detection of low-abundance proteins.
Chemiluminescence also offers a wide dynamic range, making it suitable for quantifying the relative abundance of different proteins within a sample. Additionally, this detection method is relatively straightforward and cost-effective, making it a popular choice for many researchers.
However, it is important to note that chemiluminescence has some limitations. The emitted light produced during the reaction is transient and has a short duration, requiring immediate detection. Furthermore, chemiluminescent signals can fade over time, impacting the ability to capture and analyze results accurately.
Fluorescence
Fluorescence detection is another widely used method in Western blotting. It involves the use of antibodies labeled with fluorescent dyes that emit light when excited by a specific wavelength of light. Specialized imaging systems or fluorescence microscopes can detect the emitted light.
Fluorescence detection offers several advantages, including high sensitivity and a wide dynamic range. It allows for multiplexing, which enables the simultaneous detection of multiple target proteins using different fluorescent dyes. This technique is particularly useful when studying protein-protein interactions or assessing the expression of multiple proteins within a sample.
If required, one can capture and analyze fluorescence signals at a later time, unlike chemiluminescence, which is unstable. However, the use of fluorescent dyes may require specialized equipment and additional optimization steps. Additionally, background noise can sometimes be an issue when working with fluorescence, requiring careful optimization to achieve reliable and accurate results.
To summarize, both chemiluminescence and fluorescence are valuable protein detection methods in Western blotting, each with its own set of advantages and considerations. Researchers should carefully evaluate their experimental requirements and choose the method that best aligns with their specific needs.
Below is a table summarizing the key differences between chemiluminescence and fluorescence detection methods.
| Chemiluminescence | Fluorescence |
|---|---|
| High sensitivity | High sensitivity |
| Wide dynamic range | Wide dynamic range |
| Transient signal | Stable signal |
| Immediate detection required | Flexible detection timing |
| Signal fading over time | Potential background noise |
| Straightforward and cost-effective | May require specialized equipment |
Visualization and Analysis of Western Blot Results
When it comes to Western blotting, visualizing and analyzing the results is a critical step in understanding protein expression and drawing accurate conclusions from your experiments. In this section, we will explore techniques such as result visualization, band quantification, and data analysis to equip you with the necessary skills to interpret and analyze your Western blot data effectively.
Image Capture
One of the first steps in analyzing Western blot results is capturing clear and high-quality images of your blot. By using appropriate imaging equipment and settings, you can ensure accurate representation of the protein bands. We recommend taking multiple exposures to capture both high- and low-intensity bands
Result Visualization
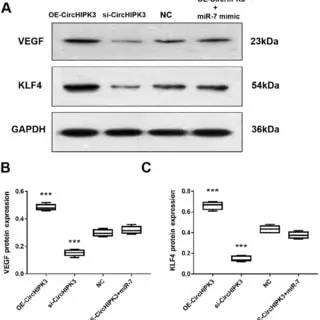
This is a Western blot results, Here’s a detailed interpretation:
Panel A: Western Blot Bands
- VEGF (23 kDa):
- The expression of VEGF protein is clearly higher in the “OE-CircHIPK3” group compared to the other conditions.
- In the “si-CircHIPK3” group, VEGF expression is significantly reduced compared to the “NC” (negative control).
- The “miR-7 mimic” condition appears to show reduced VEGF expression compared to the NC, suggesting a potential downregulatory effect of miR-7 on VEGF.
- KLF4 (54 kDa):
- KLF4 expression is significantly higher in the “OE-CircHIPK3” group.
- In the “si-CircHIPK3” group, KLF4 expression is drastically reduced, indicating that CircHIPK3 positively regulates KLF4 expression.
- Similar to VEGF, the “miR-7 mimic” group shows decreased KLF4 levels, suggesting a regulatory role of miR-7 on KLF4.
- GAPDH (36 kDa):
- GAPDH is used as a loading control to ensure equal protein loading across all lanes. The bands appear consistent, confirming proper loading and normalization.
Panel B: VEGF Quantification
- The boxplot quantifies VEGF protein levels across conditions.
- “OE-CircHIPK3”: Significantly higher VEGF expression.
- “si-CircHIPK3”: Significantly reduced VEGF expression.
- “miR-7 mimic”: VEGF expression is lower than NC, supporting the downregulatory effect.
Panel C: KLF4 Quantification
- Similar to VEGF, the boxplot shows:
- “OE-CircHIPK3”: Significantly higher KLF4 expression.
- “si-CircHIPK3”: Drastically lower KLF4 expression.
- “miR-7 mimic”: Lower KLF4 levels compared to NC, again consistent with a regulatory role of miR-7.
Interpretation:
- CircHIPK3 appears to positively regulate VEGF and KLF4 expression.
- Overexpression (OE-CircHIPK3) increases VEGF and KLF4 levels.
- Silencing (si-CircHIPK3) decreases VEGF and KLF4 levels.
- miR-7 has a potential downregulatory effect on both VEGF and KLF4.
- The miR-7 mimic reduces the expression of these proteins.
This suggests that the CircHIPK3/miR-7 axis might play a critical role in regulating VEGF and KLF4, which could be relevant to processes like angiogenesis or cancer biology.
Band Quantification
Quantifying the intensity of protein bands is essential for comparing protein expression levels between samples and conditions. Specialized software or image analysis tools can achieve this, providing accurate and reproducible quantification.
Data Analysis
Data analysis plays a crucial role in Western blot interpretation. Statistical analysis, such as comparing means or performing t-tests, allows you to determine if differences in protein expression are statistically significant. Additionally, graphing and plotting the data can provide a visual representation of your results.
By employing these techniques in result visualization, band quantification, and data analysis, you will be able to extract meaningful insights from your Western blot data. Let’s now take a look at a sample table demonstrating the quantification of protein bands:
| Sample | Protein A Intensity | Protein B Intensity |
|---|---|---|
| Control | 100 | 80 |
| Treatment | 150 | 120 |
In the table above, we can observe the quantified intensity values for Protein A and Protein B in control and treatment samples. This quantitative analysis allows us to compare the protein expression between the two conditions, providing valuable insights into the effectiveness of the treatment.
By mastering the techniques of result visualization, band quantification, and data analysis, you will be able to unlock the full potential of Western blotting and make confident conclusions based on your experimental data.
Troubleshooting and Common Pitfalls in Western Blotting
In the process of Western blotting, researchers may encounter various challenges and errors that can affect the accuracy and reliability of their results. Understanding common troubleshooting scenarios and pitfalls is essential for optimizing Western blot experiments. By addressing these issues, researchers can enhance their technique, improve data quality, and ensure successful protein analysis.
Common Errors in Western Blotting
When performing Western blotting, several common errors can occur. Identifying and resolving these errors early on is crucial to obtaining accurate and reproducible results:
- Poor sample preparation: Inadequate protein extraction or inappropriate denaturation can lead to weak or nonexistent protein bands on the blot.
- Inefficient transfer: Insufficient transfer of proteins from the gel to the membrane can result in weak or poorly defined bands, making detection challenging.
- Nonspecific binding: Incomplete blocking or improper antibody incubation can cause nonspecific binding, leading to high background noise and inaccurate protein detection.
- Inaccurate data analysis: Improper quantification or misinterpretation of band intensities can compromise the reliability of Western blot results.
Troubleshooting Tips and Optimization
To overcome these common errors and optimize Western blot experiments, researchers can follow these troubleshooting tips:
- Optimize sample preparation: Ensure proper protein extraction and denaturation by using effective lysis buffers and optimizing sample loading concentrations.
- Improve transfer efficiency: To improve transfer efficiency, choose the appropriate transfer method and optimize transfer conditions, such as voltage and duration, for the specific experimental setup.
- Enhance blocking and antibody incubation: Utilize optimized blocking agents and prolong the incubation time with primary and secondary antibodies to minimize nonspecific binding.
- Implement accurate data analysis: Use reliable software for band quantification and statistical analysis to ensure precise and consistent data interpretation.
By troubleshooting common errors and following optimization strategies, researchers can enhance the reliability and reproducibility of their Western blotting experiments, ultimately leading to more robust protein analysis.
| Error | Cause | Solution |
|---|---|---|
| Weak or nonexistent protein bands | Poor sample preparation | Optimize protein extraction and denaturation |
| Weak or poorly defined bands | Inefficient transfer | Optimize transfer conditions |
| High background noise | Nonspecific binding | Improve blocking and antibody incubation |
| Inaccurate band quantification | Improper data analysis | Implement accurate data analysis methods |
Advanced Techniques and Future Developments in Western Blotting
As Western blotting continues to be a fundamental technique in protein analysis, researchers are continually exploring advanced methods and anticipating future developments in the field. This section delves into some of the exciting advancements that are shaping the future of Western blotting.
Multiplexing
One notable advancement in Western blotting is the implementation of multiplexing techniques. Multiplexing allows for the detection of multiple proteins simultaneously, saving time and sample material. Researchers can assess protein expression levels within a single blot, enabling a comprehensive analysis of complex biological samples. This technique has opened new doors in biomarker discovery and understanding complex protein interactions.
Quantitative Analysis
The ability to perform quantitative analysis in Western blotting is a crucial advancement in protein analysis. It allows researchers to accurately measure protein expression levels and compare samples quantitatively. Quantitative Western blotting techniques, such as digital imaging and image analysis software, provide precise measurements and enable reliable quantification of protein bands. This quantitative approach enhances the accuracy and reproducibility of Western blotting experiments.
Novel Detection Methods
The development of novel detection methods has significantly expanded the possibilities in Western blotting. Innovations such as proximity ligation assay (PLA) and single-molecule detection techniques have revolutionized protein analysis. These advanced detection methods offer increased sensitivity and specificity, enabling the detection of low-abundance proteins and facilitating more precise quantification. They also provide insights into post-translational modifications and protein-protein interactions.
These advanced Western blotting techniques and future developments hold great promise for protein analysis. They enable researchers to delve deeper into the intricacies of cellular processes, identify novel biomarkers, and gain a more comprehensive understanding of disease mechanisms.
Solidifying Western blotting’s essential role in protein analysis, these advancements unlock new possibilities and drive scientific progress.
| Advanced Techniques | Benefits |
|---|---|
| Multiplexing | Simultaneous detection of multiple proteins Time and sample-saving Enhanced biomarker discovery |
| Quantitative Analysis | Precise measurement of protein expression levels Reliable quantification Improved reproducibility |
| Novel Detection Methods | Increased sensitivity and specificity Detection of low-abundance proteins Insights into post-translational modifications |
Conclusion
In conclusion, mastering the technique of Western blotting is essential for any researcher or laboratory practitioner involved in protein analysis. The comprehensive guide provided in this article has covered the key concepts and steps involved in this critical lab technique.
By understanding how to read a Western blot effectively, researchers can confidently interpret protein levels and obtain accurate results. The process of sample preparation, gel electrophoresis, protein transfer, blocking, antibody incubation, and protein detection methods were all explored in detail, equipping readers with the necessary knowledge to optimize their experiments.
Furthermore, this guide addressed common troubleshooting scenarios and pitfalls, enabling researchers to overcome challenges and ensure reliable results. Additionally, the article provided a glimpse of advanced techniques and future developments in Western blotting, highlighting the potential for further innovation in protein analysis.
Now, we encourage readers to apply the valuable knowledge they have gained in their own research endeavors or laboratory practice.
By incorporating the techniques and insights discussed in this article, researchers can enhance the accuracy and reliability of their Western blot experiments, contributing to advancements in various fields of study.


[…] electrophoresis is a powerful technique for DNA, RNA, and protein separation. However, like any scientific method, it is not without its challenges. In this section, we will […]