As we dive into the complexities of breast cancer, it is imperative to highlight the pivotal role of epigenetic changes in its development. Among these modifications, DNA methylation analysis has emerged as a critical tool in unraveling the malignant codes embedded within our genes.
By delving into breast cancer epigenetics, scientists are uncovering layers of control that go beyond our DNA sequence, influencing the disease in ways that were once hidden from our understanding.
This burgeoning field leverages intricate biomolecular tools to map out patterns that may predict the trajectory of breast cancer, equipping clinicians with the insight to tailor more effective treatment plans.
As we stand on the precipice of a new era in precision medicine, the knowledge gained from studying DNA methylation not only demystifies the enigma of breast cancer but also holds the promise of revolutionary breakthroughs in patient care.
Key Takeaways
- Epigenetic changes, such as DNA methylation, play a significant role in the development of breast cancer.
- DNA methylation analysis offers insights into the progression and potential treatment pathways for breast cancer.
- Advancing our understanding of breast cancer epigenetics can lead to more personalized and effective therapies.
- Research in DNA methylation is crucial for the early detection and prognosis of breast cancer.
- The study of epigenetic patterns is a gateway to unlocking novel diagnostic and therapeutic strategies.
Introduction to Epigenetics and Breast Cancer
The conversation about breast cancer often revolves around genetic mutations, but there is another layer to this complex picture: epigenetic regulation. Epigenetics, a field at the intersection of genetics and environmental influences, is shining a new light on breast cancer risk factors and progression. Through modifications that control gene activity without changing the DNA sequence, epigenetics is instrumental in normal development and cellular function.
Cancer epigenomics, a sub-field of epigenetics, examines how epigenetic changes such as DNA methylation, histone modification, and non-coding RNA ( like micro RNAs) interactions can disrupt cellular homeostasis, potentially leading to cancer development. The regulation of genes involved in cell growth, repair, and death is fine-tuned by these epigenetic processes, which can become deregulated and contribute to tumorigenesis.
Among the well-studied breast cancer risk factors, family history certainly plays a part, yet epigenetics provides insights into how the environment and lifestyle choices may also dictate breast cancer risk. Understanding these modifications is not just about demystifying disease pathways but forms the backbone of early detection and personalized medicine strategies. The scope of epigenetics in breast cancer research promises a future where prevention and therapy are tuned to the individual signature of epigenomic alterations.
Epigenetics does not change the genetic code; it changes how that code is read by cells, and those changes can be powerful enough to cause diseases like breast cancer.
Epigenomic perspectives on breast cancer
By mapping the epigenomic landscape of breast cancer, researchers are beginning to uncover patterns that might predict disease progression, influence responsiveness to certain treatments, and offer new avenues for therapeutic intervention. Personalized health care hinges on recognizing these patterns and acknowledging that the epigenomic profile of breast cancer is as critical as the genetic mutations it harbors.
- Epigenetic changes, though reversible, can have long-lasting effects on gene expression.
- Studying the epigenetic landscape of breast tissue can reveal how certain genes become aberrantly activated or silenced in cancer.
- Lifestyle factors, such as diet and exposure to chemicals, can induce epigenetic changes, potentially influencing breast cancer risk.
In the era of precision medicine, epigenetic regulation is more than a contributing factor to breast cancer; it’s a key player in the field of cancer epigenomics, opening doors to understanding and combating this complex disease.
The Basics of DNA Methylation
The intricate world of epigenetics is marked by numerous molecular mechanisms, with DNA methylation standing at the forefront as a vital epigenetic modification. Deepening our understanding of this process sheds light on its crucial role in gene regulation, development, and the onset of various diseases, including cancer.
What is DNA Methylation?
DNA methylation is a biochemical process that involves the methylation of cytosine bases within the DNA molecule, specifically adding a methyl group to the 5-carbon of the cytosine ring. This addition commonly occurs in a CpG dinucleotide context, where a cytosine nucleotide is followed by a guanine nucleotide. The presence of this epigenetic tag can lead to epigenetic silencing of genes, essentially turning them off when the methylated cytosines are located in gene promoter regions.
Enzymes Involved in DNA Methylation
The enzymes primarily responsible for adding methyl groups to DNA are known as DNA methyltransferases (DNMTs). These molecular architects are tightly regulated to maintain the delicate balance of DNA methylation patterns that govern gene expression. A family of DNMTs, including DNMT1, DNMT3A, and DNMT3B, collaborates to modify the epigenetic landscape, with DNMT1 maintaining methylation patterns after DNA replication, and DNMT3A and DNMT3B establishing new methylation marks.
Through the careful orchestration of DNA methyltransferases, cells can control the activation and repression of genes, an essential requirement for normal development and cellular differentiation. As research continues to reveal more about these enzymes, their role in natural processes, and their implications in the pathogenesis of diseases, we unlock further potential for therapeutic interventions aimed at modulating DNA methylation for disease treatment.
DNA Methylation in Breast Cancer
At the molecular heart of breast cancer lies a phenomenon known as hypermethylation in cancer, which dramatically alters the landscape of gene expression. This specific pattern of DNA methylation in breast cancer cells stands in stark contrast to that of healthy breast tissue, highlighting a foundational difference in the progression of this disease. Among the most significant consequences of hypermethylation is its effect on tumor suppressor genes.
Tumor suppressor genes, which normally act as the body’s defense against uncontrolled cell growth, can become inactivated through hypermethylation. This silencing is a critical step in the development of cancer, as it removes the natural constraints that prevent the proliferation of malignant cells. Consequently, the unchecked activity of oncogenes drives the progression of the disease, steering cells towards a tumorous state.
The interplay between the silencing of tumor suppressor genes and the activation of oncogenes through DNA methylation is a hallmark of the pathogenesis of breast cancer.
The current understanding of this relationship emphasizes the delicate balance between these genetic elements. When the balance is disrupted by epigenetic changes, breast cancer can take hold and advance. The research community continues to decode the complex mechanisms by which hypermethylation contributes to the pathophysiology of breast cancer, aiming to develop targeted treatments capable of reactivating these critical genes.
- The patterns of DNA methylation in breast cancer differ fundamentally from normal breast tissue.
- Hypermethylation leads to the inactivation of tumor suppressor genes, liberating oncogenes to instigate cancer growth.
- Understanding the epigenetic landscape of breast cancer provides new avenues for treatment and intervention.
How DNA Methylation Influences Gene Expression
Central to the discussion of gene silencing in cancer is the intricate process of DNA methylation, particularly at the promoter regions of genes. This form of epigenetic modification plays a pivotal role not only in cellular development and genetic stability but also in the insidious development of diseases such as cancer. The process of promoter methylation often has a profound effect on gene expression, as it can silence genes that are essential in maintaining normal cell function and suppressing tumor growth.
The methylation of promoter regions is a particularly potent form of transcriptional regulation, effectively silencing genes by preventing the transcriptional machinery from accessing the DNA. This silencing has far-reaching implications, including the inactivation of genes involved in DNA repair, cell cycle regulation, and apoptosis—all of which can contribute to the onset and progression of breast cancer when disrupted.
When promoter methylation leads to gene silencing, it is like switching off the molecular safeguards that protect against unchecked cell proliferation and tumor development.
Methylated genes in breast cancer
In the context of breast cancer, the implications of silenced genes due to aberrant promoter methylation patterns are significant. The genes typically targeted in breast cancer include those that are guardians of the genome, responsible for preventing the accumulation of genetic mutations and maintaining genomic integrity.
- BRCA1 and BRCA2 genes, when methylated and silenced, contribute to a higher risk of breast and ovarian cancers due to compromised DNA repair pathways.
- The p16 gene, a regulator of the cell cycle, can be methylated and silenced, removing an important checkpoint that prevents the uncontrollable division of cells.
- MLH1, a gene involved in DNA mismatch repair, can also be inactivated through methylation, increasing the mutation rate and fostering genetic instability.
The ongoing research into the impact of promoter methylation on gene expression aims to enhance our comprehension of the intricate mechanisms that govern cancer progression. By targeting these epigenetic changes, new therapeutic strategies may be developed to reverse gene silencing and regain control over aberrant cell growth in breast cancer.
The Role of DNA Methylation in Cancer Development
Delving into the DNA of cancer cells reveals unique patterns of methylation that serve as cryptic clues to the disease’s behavior and aggressiveness. Some of these patterns are frequently seen as red flags, indicating a disruption in the normal programming of the cell. Specifically, in breast cancer, these aberrant methylation events have profound implications for disease development and the appearance of epigenetic biomarkers.
Abnormal Methylation Patterns in Cancer
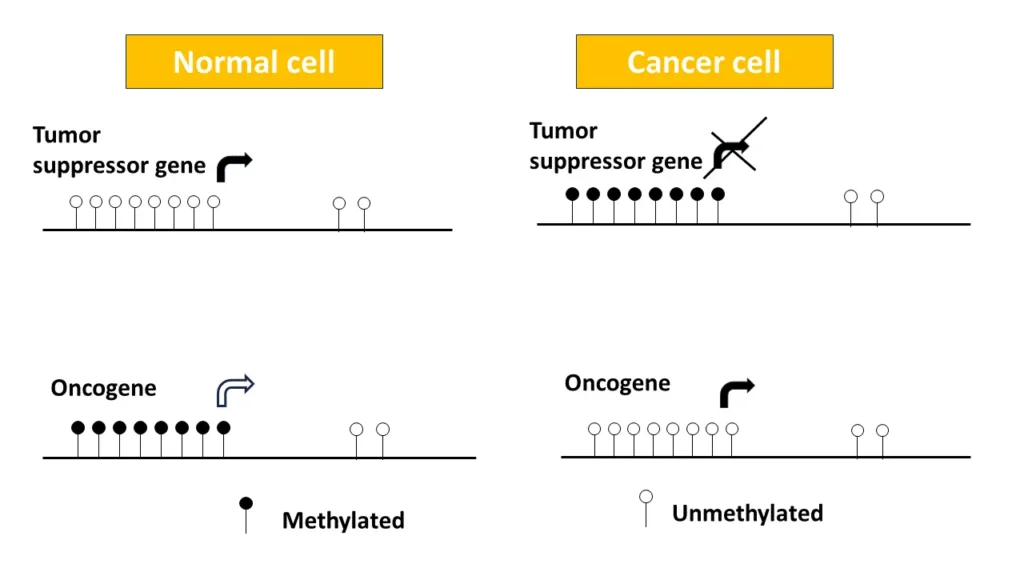
Aberrant DNA methylation, particularly CpG island methylation, is synonymous with the onset and progression of cancerous cells. This paradoxical situation of global hypomethylation alongside local hypermethylation within CpG islands leads to genomic instability and the alteration of gene expression. Global hypomethylation often triggers chromosomal abnormalities and the reactivation of transposable elements, while CpG island hypermethylation can result in the silencing of critical tumor suppressor genes.
Understanding these methylation patterns is key to grasping the complexities of cancer biology and has direct implications on our approach to treating this far-reaching disease.
While the mechanisms are intricate, the repercussions are clear: these epigenetic changes can serve as early warning signs, heralding breast cancer’s arrival and guiding strategies for interception and therapeutic intervention. Continuous exploration of these patterns enhances the precision of our biological insights and fortifies the battle against cancer.
Genes Affected by Methylation in Breast Cancer
In breast cancer, the list of genes affected by methylation is not only extensive but also deeply significant to the disease’s trajectory and the risk of breast cancer metastasis. Tumor suppressor genes are frequent targets of hypermethylation in these cases, leading to decreased expression and the loss of protective functions within the cell.
- Commonly affected genes include BRCA1, a pivotal gene in DNA repair mechanisms, which, when hypermethylated, correlates with increased cancer severity.
- Gene p16, a cyclin-dependent kinase inhibitor, when silenced by methylation, disrupts cell cycle control, allowing unregulated proliferation.
- The DNA repair gene MLH1, when hypermethylated, contributes to genetic instability, a trademark of many cancers.
These genes, among others, are shaping the way we understand epigenetic contributions to cancer. By pinpointing which genes are targeted by methylation, we not only understand more about how breast cancer develops but also how it may spread, offering vital knowledge for potential intervention. The correlation of specific methylation patterns with disease prognosis and metastatic potential paves the way for the use of these marks as actionable epigenetic biomarkers.
Identifying Breast Cancer Subtypes Through Methylation Profiling
The complexity of breast cancer heterogeneity has long presented a challenge to the medical community. With the advent of epigenetic diagnostics, we are now beginning to see a clearer picture of the various subtypes of this multifaceted disease. Through detailed methylation profiling, scientists can decipher the particular methylation signatures that distinguish one subtype of breast cancer from another.
At the heart of this process lies the ability to scrutinize changes in DNA methylation patterns across different cancer cells. Variations in methylation not only reveal critical information about gene expression but also offer clues about the aggressiveness of the tumor and its likely response to treatment.
Methylation profiling stands as a beacon of hope for improving the accuracy of breast cancer diagnosis, ultimately leading to more personalized and effective therapeutic strategies.
Distinguishing between breast cancer subtypes is essential for developing targeted treatment plans, and methylation signatures serve as the key to unlocking this specificity. By examining these patterns, clinicians can gain insight into the tumor’s biology and craft a treatment regimen that is finely tuned to the individual patient’s cancer characteristics.
- Identifies unique methylation patterns that correlate with specific breast cancer subtypes
- Enhances prediction of disease progression and potential for metastasis
- Guides personalized treatment decisions based on the epigenetic profile of the tumor
- Improves prognosis by enabling earlier and more accurate detection of breast cancer
- Opens up avenues for new and innovative epigenetic diagnostics in oncology
As we deepen our grasp of breast cancer heterogeneity, epigenetic diagnostics become integral in not just understanding the disease but also in combating it. The potential of methylation profiling to redefine the landscape of cancer treatment is profound, paving the way for a future where every breast cancer patient receives care that is as unique as their methylation signature.
Environmental Factors Contributing to Methylation Changes
Our environment and lifestyle choices are more intricate than we may realize, especially concerning our health. Recent evidence in the field of environmental epigenetics indicates that various external factors can induce epigenetic alterations which could, in turn, increase the risk of developing diseases such as breast cancer. Investigating these complex interactions highlights how our daily lives may influence our epigenetic landscape and predispose us to health challenges.
Lifestyle and Dietary Factors
The connection between our diet, level of physical activity, and overall health is well-established, but less known is the effect of these aspects on our epigenome. Poor dietary choices, sedentary lifestyles, and obesity are not just risk factors for various chronic diseases but also potential instigators of epigenetic alterations. High-fat diets, for instance, have been associated with changes in DNA methylation patterns that could exacerbate the risk of cancer development.
Engaging in regular physical activity and adopting a balanced diet rich in folate and other methylation-supporting nutrients may help to maintain normal DNA methylation processes and potentially deter carcinogen exposure-related changes.
- Increased intake of green leafy vegetables can support efficient DNA methylation through folate and other B vitamins.
- Restrictive fad diets may unintentionally impact the epigenetic mechanisms governing gene expression.
- Obesity, often linked with poor dietary and exercise habits, can lead to systemic inflammation and disrupt normal methylation pathways.
Exposure to Endocrine Disrupting Chemicals
A growing body of research links carcinogen exposure to adverse epigenetic modifications. Chemicals present in plastics, pesticides, and other pollutants act as endocrine disruptors; they mimic or interfere with the normal functions of hormones in the body. Such disruptions can result in DNA methylation changes that, over time, may increase the likelihood of breast cancer onset.
Chronic exposure to a class of such chemicals, known as xenoestrogens, is particularly concerning due to its potential effects on methylation and breast cancer risk.
- Routine use of certain plastics, which release bisphenol A (BPA), an endocrine disruptor, can lead to epigenetic changes.
- Pesticides containing organochlorines, with their persistent nature in the environment, are shown to affect DNA methylation patterns.
- Phthalates, commonly found in cosmetics and personal care products, are another group of chemicals associated with epigenetic disruptions.
The complexity and pervasiveness of environmental epigenetics underscore the need for informed public health strategies that address not only genetic but also epigenetic risk factors. Understanding the intricate networks between diet, lifestyle, environmental exposures, and our epigenome will be crucial in developing practical preventative measures against diseases like breast cancer influenced by epigenetic alterations.
Conclusion
Throughout our exploration of the intricate links between DNA methylation and breast cancer, we have uncovered the profound influence of epigenetic processes on both the development and the potential management of this disease. The implications of methylation patterns extend beyond mere cellular changes, steering the directions for breast cancer prevention and therapeutic innovation. It’s within these complex biological signatures that the future of precision oncology is being written, offering hope for treatments that are as unique as the individual’s genetic and epigenetic makeup.
The potential of DNA methylation as a biomarker for diagnosis and prognosis underscores the strides being taken in the realm of epigenetic therapy. As research persists, we inch closer to the ideal of personalized medicine where treatment strategies are tailored to the patient’s specific disease profile. The seamless integration of epigenetic findings into clinical applications heralds a promising transition in oncology—a transition where precision supersedes one-size-fits-all approaches, harmonizing treatment with patients’ unique epigenetic landscapes.
In the mission towards optimal health outcomes, understanding the impacts of lifestyle and environmental factors on epigenetic alterations is indispensable. By incorporating this knowledge into preventive measures, individuals can make empowered choices that potentially reduce their risk of developing breast cancer. In unison with medical advancements, such proactive steps in breast cancer prevention solidify our defense against this pervasive ailment. Ultimately, as we navigate through these scientific revelations, it becomes abundantly clear that the key to conquering breast cancer may indeed lie within the intricate world of epigenetics.
FAQ
What is DNA methylation and why is it significant in breast cancer?
DNA methylation is an epigenetic mechanism that adds a methyl group to the DNA, affecting gene expression without changing the DNA sequence itself. It is significant in breast cancer because it can lead to the silencing of tumor suppressor genes and promote cancer development and progression.
How does epigenetic regulation contribute to breast cancer?
Epigenetic regulation, including DNA methylation, histone modification, and non-coding RNA, can alter gene expression in cells. Dysregulation of these epigenetic processes can disrupt normal cell function and contribute to the initiation and progression of breast cancer by affecting breast cancer risk factors and detection.
What are DNA methyltransferases and their role in DNA methylation?
DNA methyltransferases are enzymes that facilitate the process of adding a methyl group to the DNA, primarily at cytosine bases. They play a crucial role in the maintenance of the methylation pattern during cell division and are integral in controlling gene expression levels in cells.
In what way does hypermethylation affect breast cancer?
Hypermethylation often targets tumor suppressor genes, leading to their silencing and loss of function. This can contribute to uncontrolled cell growth and division, a characteristic of cancer, including breast cancer.
Can you explain how DNA methylation influences gene expression in breast cancer?
In breast cancer, DNA methylation typically occurs at promoter regions of genes, leading to gene silencing. This suppresses the expression of genes crucial for cancer prevention, contributing to the onset and progression of the disease while also affecting transcriptional regulation.
What are abnormal methylation patterns observed in cancer?
Abnormal methylation patterns in cancer include global hypomethylation, which may lead to chromosomal instability and CpG island hypermethylation, potentially resulting in the silencing of tumor suppressor genes and activation of oncogenes, contributing to cancer development.
How does methylation profiling help in identifying breast cancer subtypes?
Methylation profiling can reveal specific methylation signatures associated with different breast cancer subtypes. This information can help in understanding the prognosis of the disease and in tailoring more precise treatment strategies for patients.
Can environmental factors lead to methylation changes tied to breast cancer?
Yes, environmental factors such as lifestyle, diet, physical activity, and exposure to endocrine-disrupting chemicals can induce epigenetic alterations like DNA methylation changes. These can influence an individual’s risk of developing breast cancer.