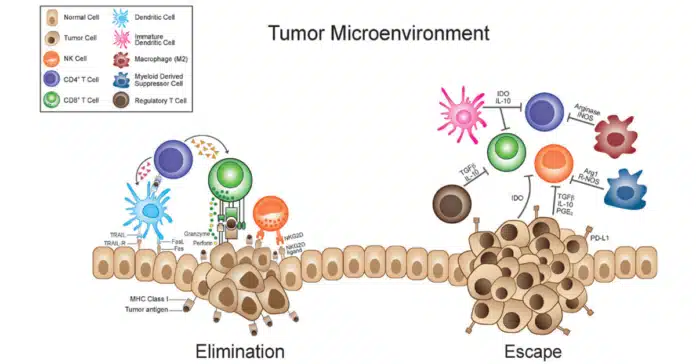
The tumor microenvironment (TME) is a dynamic and complex network of cancer cells, stromal cells, immune cells, and molecular factors that surround and interact with a tumor. This ecosystem plays a pivotal role in cancer progression, influencing tumor growth, metastasis, and resistance to therapies. Understanding the TME is crucial, as it opens new avenues for developing targeted cancer treatments.
In this blog post, we’ll explore the key components of the TME, the molecular pathways driving its behavior, and innovative strategies to study and target it, highlighting its significance in advancing cancer therapies.
What Is the Tumor Microenvironment?
The tumor microenvironment (TME) consists of cancer cells and their surrounding support network, including stromal cells, immune cells, and endothelial cells. This environment plays a critical role in tumor progression by fostering cellular interactions and communication, known as tumor heterogeneity and crosstalk.
Key processes such as angiogenesis (formation of new blood vessels), hypoxia (low oxygen levels), and chronic inflammation drive tumor growth, metastasis, and resistance to treatments
Cellular Components of the Tumor Microenvironment
The tumor microenvironment (TME) is composed of various cellular players that interact to influence cancer progression and therapy responses. Below are the key cellular players in the TME:
.Cancer-Associated Fibroblasts (CAFs)
CAFs are one of the most abundant cell types in the TME. These are modified fibroblasts that actively promote tumor growth and metastasis through several mechanisms:
- Extracellular Matrix (ECM) Remodeling: CAFs produce ECM proteins like collagen, fibronectin, and laminin, creating a structural scaffold that supports cancer cell invasion.
- Secretion of Growth Factors: They release growth factors such as TGF-β, FGF, and VEGF, which stimulate tumor growth and angiogenesis.
- Promoting Therapy Resistance: CAFs can create a physical and biochemical barrier that shields tumor cells from therapeutic agents.
Immune Cells in Tumor Microenvironment
The TME is infiltrated by various immune cells, many of which are co-opted by cancer to evade immune detection:
- Macrophages:
- Tumor-associated macrophages (TAMs) can adopt two phenotypes:
- M1 Macrophages: Pro-inflammatory and anti-tumorigenic.
- M2 Macrophages: Anti-inflammatory and pro-tumorigenic, supporting immune evasion and tissue remodeling.
- TAMs predominantly exhibit the M2 phenotype in the TME, promoting tumor growth.
- Tumor-associated macrophages (TAMs) can adopt two phenotypes:
- T Cells:
- Cytotoxic T Cells (CTLs): Normally destroy cancer cells but are often suppressed in the TME.
- Regulatory T Cells (Tregs): Suppress immune responses and help the tumor evade detection.
- Natural Killer (NK) Cells:
- These are innate immune cells that target abnormal cells, but their function is often inhibited in the TME by factors secreted by tumor cells.
- Dendritic Cells (DCs):
- Critical for antigen presentation, but their activity is often impaired in the TME, reducing immune activation.
Endothelial Cells
Endothelial cells form the inner lining of blood vessels and are pivotal in angiogenesis, the process of forming new blood vessels.
- Role in Tumor Growth: Tumors release VEGF (vascular endothelial growth factor), stimulating endothelial cells to form abnormal, leaky blood vessels that provide nutrients to the tumor.
- Facilitation of Metastasis: These leaky vessels enable cancer cells to enter the bloodstream and spread to other organs.
Pericytes
Pericytes are cells that wrap around endothelial cells, stabilizing blood vessels.
- Role in Angiogenesis: In the TME, pericytes support the development of irregular vasculature.
- Tumor Invasion: Their interaction with endothelial cells contributes to vascular permeability, aiding cancer cell dissemination.
Adipocytes
Adipocytes, or fat cells, are increasingly recognized as active contributors to the TME, especially in cancers like breast and ovarian cancer.
- Energy Supply: They provide fatty acids as an energy source for rapidly proliferating cancer cells.
- Secretion of Adipokines: Adipocytes release hormones and cytokines, such as leptin and adiponectin, that can promote cancer growth.
- Facilitation of Metastasis: Adipocytes contribute to the local invasion of cancer cells by releasing matrix metalloproteinases (MMPs).
Mesenchymal Stem Cells (MSCs)
MSCs are multipotent stromal cells present in the TME, recruited from bone marrow or local tissues.
Promotion of Therapy Resistance: They can alter the TME to shield cancer cells from chemotherapeutic agents.
Pro-tumorigenic Role: MSCs secrete cytokines and growth factors that enhance tumor cell proliferation, migration, and survival.
Molecular Pathways Driving the Tumor Microenvironment
Several molecular pathways are crucial in shaping the tumor microenvironment (TME) and supporting tumor growth, metastasis, and immune evasion.
1. VEGF Signaling and Angiogenesis
Vascular Endothelial Growth Factor (VEGF) is a key signaling molecule that stimulates angiogenesis, the process of new blood vessel formation. These newly formed blood vessels supply tumors with oxygen and nutrients, facilitating their growth and enabling metastasis to distant organs.
2. TGF-beta Pathway
The Transforming Growth Factor-beta (TGF-beta) signaling pathway plays a significant role in promoting cancer cell invasion and metastasis. It supports tumor progression by inducing epithelial-to-mesenchymal transition (EMT), which enhances the mobility of cancer cells and their ability to invade surrounding tissues.
3. PD-1/PD-L1 Axis
The PD-1/PD-L1 axis is a critical immune checkpoint mechanism in the TME. Tumor cells express PD-L1, which binds to the PD-1 receptor on T-cells, effectively suppressing the immune response. This immune evasion allows tumors to escape detection and destruction by the body’s immune system.
These molecular pathways not only drive tumor progression but also present potential targets for therapeutic interventions aimed at disrupting the TME.
Challenges and Opportunities in Targeting the Tumor Microenvironment
Targeting the tumor microenvironment (TME) presents both significant challenges and exciting opportunities for improving cancer treatment outcomes.
1. Therapy Resistance
The TME can contribute to therapy resistance by creating physical and chemical barriers that prevent effective drug delivery, altering drug metabolism, or by enabling tumor cells to adapt to stress. Additionally, the presence of immune suppressive cells within the TME can render chemotherapy and targeted therapies less effective, leading to tumor recurrence.
2. Immune Checkpoint Inhibitors
Immune checkpoint inhibitors, such as those targeting the PD-1/PD-L1 axis, represent a major advancement in cancer immunotherapy. These therapies work by blocking the immune suppression mechanisms within the TME, enabling T-cells to attack cancer cells more effectively. Despite their success, many tumors develop resistance to these therapies, highlighting the need for improved strategies and combination treatments.
3. TME-Targeted Approache
New therapeutic strategies are focused on directly targeting the TME. Anti-angiogenic therapies aim to block the formation of blood vessels that supply tumors, while ECM remodeling therapies seek to break down the structural support that protects cancer cells. These approaches can improve the efficacy of existing treatments and make tumors more susceptible to immune attacks and chemotherapy.
While challenges remain in overcoming TME-related resistance, these innovative approaches hold promise for enhancing cancer treatment and potentially transforming clinical outcomes.
Emerging Technologies to Study and Target the Tumor Microenvironment
Advances in technology are providing new ways to study and target the tumor microenvironment (TME), offering insights into cancer biology and potential therapeutic strategies.
1. 3D Tumor Models and Organoids
3D tumor models and organoids are revolutionizing cancer research by better mimicking the complexities of the TME compared to traditional 2D cultures. These models enable researchers to study tumor behavior in a more realistic context, test new drugs, and understand how the TME influences cancer progression and therapy resistance.
2. Nanotechnology in TME-Targeted Therapy
Nanotechnology is enabling more precise delivery of therapeutic agents directly to the TME. Nanoparticles can be engineered to target specific molecules or cells within the tumor, increasing drug efficacy and minimizing side effects. This technology holds great promise for overcoming the barriers of drug resistance and improving treatment outcomes.
3. Future Directions in Tumor Microenvironment Research
As research into the TME advances, future studies will focus on understanding the molecular mechanisms that drive its dynamics. By identifying key signaling pathways and interactions within the TME, researchers aim to develop personalized therapies tailored to the unique microenvironment of individual tumors. This could lead to more effective and targeted treatments with fewer side effects.
These emerging technologies are transforming how we study and treat cancer, offering hope for more effective and personalized cancer therapies in the near future.
Conclusion
The tumor microenvironment (TME) plays a critical role in cancer progression, immune evasion, and therapy resistance. Understanding its cellular components, molecular pathways, and key processes opens up new possibilities for innovative treatments.
While challenges like therapy resistance remain, emerging technologies such as 3D tumor models, nanotechnology, and immune checkpoint inhibitors offer promising solutions.
As research advances, the ability to target and manipulate the TME for more personalized and effective therapies will be key to improving cancer treatment outcomes.
By continuing to explore the complexities of the TME, we can hope to pave the way for breakthroughs in cancer therapy that better address the unique characteristics of each tumor.