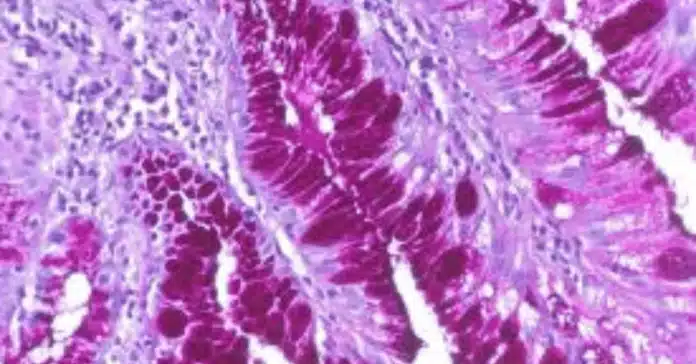
Histological staining techniques are essential tools in pathology and biomedical research, providing critical insights into the structure and composition of tissues. Among these techniques, PAS staining—short for Periodic Acid-Schiff staining—stands out due to its ability to detect carbohydrates, glycogen, mucopolysaccharides, and glycoproteins in tissue samples. This method plays a pivotal role in diagnosing various diseases, including fungal infections, glycogen storage disorders, and certain types of cancers.
But what makes PAS staining so important? Its specificity for carbohydrate-rich structures makes it invaluable in differentiating between normal and abnormal tissue components, offering essential clues for clinical diagnosis and research. By highlighting these structures with a distinct magenta coloration, pathologists can accurately identify pathological changes that might otherwise go unnoticed with routine staining methods.
Understanding the principles, procedures, and applications of PAS staining is crucial for anyone working in histology, pathology, or cancer research. Whether you’re a student, researcher, or healthcare professional, mastering this staining technique will enhance your ability to interpret tissue samples effectively.
👉 In this blog post, we will explore the fundamental principles behind PAS staining, provide a step-by-step protocol, discuss how to interpret staining results, and delve into its clinical applications. We will also cover variations like PAS-Diastase staining, troubleshoot common issues, and answer frequently asked questions to help you gain a comprehensive understanding of this essential histological technique.
The Science Behind PAS Staining
The Periodic Acid-Schiff (PAS) staining technique is based on a chemical reaction that highlights carbohydrate-rich structures in tissues. It is particularly useful for detecting glycogen, mucopolysaccharides, glycoproteins, and basement membranes. Understanding the science behind PAS staining helps explain why it is such a powerful tool in histology and pathology.
🔬 Principle of PAS Staining
The core principle of PAS staining revolves around the oxidation of carbohydrates by periodic acid. This process breaks the carbon-carbon bonds in carbohydrates, converting the hydroxyl groups (-OH) into aldehyde groups (-CHO). Once these aldehyde groups are exposed, they react with the Schiff reagent, leading to the formation of a bright magenta (pink-purple) color.
This color change occurs because the Schiff reagent, initially colorless, regains its color when it binds to the aldehyde groups. The intensity of the magenta color corresponds to the amount of carbohydrate present in the tissue.
Key Points:
- Periodic acid: Oxidizes carbohydrates, exposing aldehyde groups.
- Schiff reagent: Reacts with aldehydes, producing a magenta color.
- Magenta coloration: Indicates PAS-positive areas, highlighting carbohydrate-rich structures.
🧪 Chemical Reactions Involved
The PAS staining process involves two primary chemical reactions:
- Oxidation Reaction:
- Periodic acid (HIO₄) oxidizes vicinal diols (two hydroxyl groups on adjacent carbon atoms) in carbohydrates, forming aldehyde groups.
- Example: R-CHOH-CHOH-R’+HIO4→R-CHO+R’-CHO+H2O
- Color Development Reaction:
- The Schiff reagent, which contains decolorized basic fuchsin, reacts with the aldehyde groups to restore its magenta color.
- The intensity of the magenta color correlates with the concentration of aldehyde groups, thus reflecting the presence of carbohydrates.
🧬 Why PAS Staining is Specific for Carbohydrates
PAS staining specifically detects carbohydrate-rich molecules because of the unique ability of periodic acid to oxidize vicinal diols. Carbohydrates like glycogen, mucins, glycoproteins, and proteoglycans have these chemical groups, making them ideal targets.
Moreover, basement membranes, which contain glycoproteins and proteoglycans, show strong PAS positivity. This makes PAS staining particularly useful in diagnosing conditions where basement membrane integrity is compromised, such as certain cancers and kidney diseases.
💡 What Does PAS Staining Detect?
- Glycogen: Stored energy in cells, commonly detected in liver and muscle tissues.
- Mucins: Found in epithelial tissues and secretions; important in diagnosing adenocarcinomas.
- Basement Membranes: Structural layers beneath epithelial tissues, critical in identifying invasive tumors.
- Fungi: PAS staining highlights fungal cell walls, aiding in diagnosing infections like Candida albicans.
- Glycoproteins and Proteoglycans: Essential components of connective tissues.
🏥 Clinical Relevance of the PAS Reaction
- Cancer Diagnosis: PAS staining can detect mucin-producing tumors (e.g., adenocarcinomas) and evaluate basement membrane integrity to assess tumor invasiveness.
- Fungal Infections: The cell walls of fungi stain PAS-positive, making it useful for identifying infections, especially in immunocompromised patients.
- Glycogen Storage Diseases: The presence and amount of glycogen in tissues can indicate metabolic disorders.
- Whipple’s Disease: PAS-positive macrophages in the intestinal mucosa help diagnose this rare bacterial infection.
Step-by-Step PAS Staining Protocol
Performing PAS staining accurately is essential for obtaining clear, reliable results that highlight carbohydrate-rich structures in tissues. This section outlines a comprehensive step-by-step protocol, including the required materials, detailed procedures, and essential precautions to ensure optimal staining outcomes.
🧪 Materials and Reagents Required
Before beginning the PAS staining procedure, gather the following materials and reagents:
- Materials:
- Tissue sections (formalin-fixed, paraffin-embedded) on microscope slides
- Coplin jars or staining racks
- Distilled water
- Light microscope
- Reagents:
- Periodic acid solution (0.5%–1%)
- Schiff reagent
- Hematoxylin (optional, for counterstaining)
- Sulfurous acid or 0.5% sodium metabisulfite solution (to remove excess Schiff reagent)
- Running tap water
- Ethanol (70%, 95%, and absolute)
- Xylene
- Mounting medium and coverslips
🔬 Step-by-Step PAS Staining Procedure
Step 1: Deparaffinization and Hydration
- Place slides in xylene for 2–3 minutes (repeat twice) to remove paraffin.
- Hydrate tissue sections by passing them through a graded series of ethanol (100%, 95%, and 70%) for 2 minutes each.
- Rinse slides in distilled water for 5 minutes.
✅ Tip: Ensure complete deparaffinization for uniform staining.
Step 2: Oxidation with Periodic Acid
- Immerse slides in 0.5% periodic acid solution for 5–10 minutes at room temperature.
- Rinse thoroughly in distilled water for 5 minutes.
🧬 Purpose: Periodic acid oxidizes carbohydrate components, exposing aldehyde groups.
Step 3: Staining with Schiff Reagent
- Place slides in Schiff reagent for 15–20 minutes in the dark (to prevent light-induced degradation).
- Rinse in running tap water for 10 minutes until the sections turn pink.
🎨 Tip: The magenta color intensity indicates the amount of aldehyde groups present.
Step 4: (Optional) Counterstaining with Hematoxylin
- Stain with hematoxylin for 1–2 minutes to visualize nuclei.
- Rinse in tap water and blue in a weak alkaline solution (e.g., ammonia water).
🔍 Purpose: Counterstaining enhances tissue contrast and aids interpretation.
Step 5: Dehydration, Clearing, and Mounting
- Dehydrate sections through graded ethanol (70%, 95%, 100%) for 2 minutes each.
- Clear in xylene (two changes, 3 minutes each).
- Mount sections using a synthetic mounting medium and cover with coverslips.
🎯 Precautions and Safety Tips
- Handle Schiff reagent with care, as it contains potentially hazardous chemicals.
- Use proper ventilation when working with xylene and other volatile solvents.
- Protect slides from light during Schiff reagent incubation to prevent fading.
- Ensure precise timing during oxidation and staining steps to avoid over- or under-staining.
💡 Expected Results After PAS Staining
- PAS-positive structures will appear magenta (pink-purple) under the microscope.
- Nuclei (if counterstained with hematoxylin) will appear blue.
- PAS-negative areas will remain unstained or pale.
Common PAS-positive structures include:
- Glycogen in liver and muscle cells
- Basement membranes in epithelial tissues
- Fungal organisms (e.g., Candida albicans)
- Mucins in glandular epithelial cells
⚡ Quick Troubleshooting Tips
- Weak staining: Ensure proper oxidation and check Schiff reagent quality.
- Overstaining: Reduce Schiff reagent incubation time or adjust periodic acid concentration.
- Uneven staining: Confirm complete deparaffinization and even reagent distribution.
👉 In the next section, we will explore how to interpret PAS staining results, distinguish between PAS-positive and PAS-negative tissues, and discuss the clinical significance of these findings.
Interpretation of PAS Staining Results
The interpretation of PAS staining results is crucial for understanding tissue structure, diagnosing diseases, and identifying pathological changes. After successfully performing the PAS staining protocol, it’s essential to accurately analyze the color patterns and intensities observed under the microscope. The magenta (pink-purple) coloration signifies the presence of carbohydrate-rich structures, and its distribution and intensity can provide vital diagnostic insights.
🎨 Understanding PAS-Positive and PAS-Negative Results
- PAS-Positive Results:
- Structures that stain magenta or pink-purple are considered PAS-positive.
- The intensity of the staining reflects the amount and type of carbohydrates present.
- Common PAS-positive structures include:
- Glycogen in liver and muscle tissues.
- Basement membranes beneath epithelial tissues.
- Mucins in glandular epithelium.
- Fungal cell walls (e.g., Candida albicans).
- Glycoproteins and proteoglycans in connective tissues.
- PAS-Negative Results:
- Areas that remain unstained or pale indicate an absence of detectable carbohydrates.
- PAS-negative results are normal in tissues lacking carbohydrate-rich structures.
- If PAS positivity is expected but absent, it may indicate technical errors or tissue abnormalities.
🔬 Key Structures and Their Staining Patterns
| Structure | PAS Staining Appearance | Clinical Significance |
|---|---|---|
| Glycogen | Diffuse magenta in cytoplasm | Indicates glycogen storage or metabolism disorders. |
| Basement membranes | Sharp magenta lines | Essential for detecting tumor invasiveness and glomerular diseases. |
| Mucins | Intense magenta in glands | Helps identify mucin-producing tumors like adenocarcinomas. |
| Fungi | Magenta fungal cell walls | Critical for diagnosing fungal infections. |
| Glycoproteins | Magenta deposits in tissues | Found in various connective tissue diseases. |
🏥 Clinical Relevance of PAS Staining Results
- Cancer Diagnosis:
- Adenocarcinomas often produce mucins that stain PAS-positive.
- Basement membrane assessment using PAS staining helps differentiate between invasive and non-invasive tumors.
- Loss or disruption of basement membranes may indicate tumor invasion.
- Infectious Diseases:
- Fungal organisms such as Candida, Aspergillus, and Histoplasma show PAS-positive cell walls, aiding in rapid diagnosis.
- Metabolic Disorders:
- Glycogen storage diseases can be identified by the accumulation of glycogen in tissues, which appears as PAS-positive regions.
- Use of diastase digestion (PAS-D) distinguishes glycogen from other PAS-positive materials.
- Whipple’s Disease:
- Identified by PAS-positive macrophages in the intestinal mucosa, essential for diagnosing this rare bacterial infection.
💡 PAS-Diastase (PAS-D) Staining for Differentiation
To confirm that PAS positivity is due to glycogen, a PAS-Diastase (PAS-D) stain is used. Diastase breaks down glycogen:
- If the magenta staining disappears after diastase treatment, the material is confirmed as glycogen.
- If staining persists, it suggests the presence of other carbohydrates, such as mucins or fungal elements.
⚡ Common Pitfalls in Interpretation and How to Avoid Them
- False Negatives:
- Incomplete oxidation or degraded Schiff reagent can lead to weak or absent staining.
- Ensure fresh reagents and proper incubation times.
- Overstaining or Background Staining:
- Prolonged exposure to Schiff reagent or inadequate washing can cause non-specific staining.
- Always rinse slides thoroughly and adhere to timing guidelines.
- Misinterpretation of PAS-Positive Structures:
- Without counterstaining (e.g., hematoxylin), distinguishing cellular details can be challenging.
- Always include a nuclear counterstain for clearer interpretation.
🔍 Microscopic Examination Tips
- Use a light microscope at 10x and 40x objectives for general examination.
- For detailed cellular analysis, use the 100x oil immersion objective.
- Evaluate staining patterns in context with clinical data and other diagnostic findings.
👉 In the next section, we will explore the clinical applications of PAS staining, including its role in diagnosing various cancers, infectious diseases, and metabolic disorders, highlighting its importance in modern pathology.
Applications of PAS Staining in Pathology
The Periodic Acid–Schiff (PAS) staining technique plays a vital role in pathology due to its ability to detect carbohydrate-rich structures in tissues. This staining method aids in the diagnosis of various diseases, including cancers, metabolic disorders, and infections. In this section, we will explore the key clinical applications of PAS staining and its relevance in modern diagnostic pathology.
🏥 1. Cancer Diagnosis and Tumor Characterization
PAS staining is essential for identifying certain cancers by detecting mucins, glycogen, and basement membranes, which are critical in tumor assessment.
- Adenocarcinomas:
- These cancers often produce mucins that stain magenta with PAS.
- Helps differentiate mucin-producing tumors (e.g., colorectal, gastric, and breast adenocarcinomas) from other tumor types.
- Basement Membrane Integrity:
- PAS highlights basement membranes, helping determine tumor invasiveness.
- Non-invasive tumors show intact basement membranes, while invasive carcinomas exhibit basement membrane disruption.
- Clear Cell Carcinomas:
- These tumors contain abundant glycogen, which appears PAS-positive.
- PAS-Diastase (PAS-D) can confirm glycogen presence by removing the staining if glycogen is present.
🦠 2. Detection of Infectious Diseases
PAS staining is highly effective in identifying fungal infections and certain bacterial diseases:
- Fungal Infections:
- Fungi such as Candida, Aspergillus, and Histoplasma have PAS-positive cell walls.
- The magenta staining of fungal elements aids in rapid and accurate diagnosis.
- Whipple’s Disease:
- Caused by Tropheryma whipplei, this disease shows PAS-positive macrophages in the small intestine’s lamina propria.
- PAS staining is essential for diagnosing this rare but serious bacterial infection.
🧬 3. Identification of Metabolic and Storage Disorders
PAS staining can reveal the accumulation of carbohydrates in various metabolic disorders:
- Glycogen Storage Diseases (GSD):
- PAS staining highlights glycogen accumulation in liver and muscle tissues.
- PAS-Diastase digestion confirms glycogen by eliminating the magenta staining if glycogen is present.
- Alport Syndrome:
- PAS staining is used to evaluate the glomerular basement membrane for abnormalities in renal biopsies.
- Essential for diagnosing hereditary nephropathies.
- Pompe Disease:
- A lysosomal storage disorder showing PAS-positive glycogen in muscle fibers.
- Critical for early diagnosis and management of this condition.
🧪 4. Liver and Kidney Pathology
PAS staining is invaluable in assessing structural changes in the liver and kidneys:
- Liver Biopsies:
- Identifies glycogen storage, aiding in diagnosing hepatocellular disorders.
- Detects alpha-1 antitrypsin deficiency through PAS-positive, diastase-resistant inclusions.
- Renal Biopsies:
- Visualizes glomerular basement membranes to assess glomerulonephritis and diabetic nephropathy.
- Thickening or splitting of membranes seen with PAS staining provides diagnostic clues.
🏃 5. Muscle Pathology
In muscle biopsies, PAS staining helps identify glycogen storage and other carbohydrate-related disorders:
- Pompe Disease (GSD type II):
- Shows PAS-positive glycogen accumulation in muscle fibers.
- McArdle Disease (GSD type V):
- Muscle biopsies reveal glycogen storage with PAS positivity, assisting in differential diagnosis.
🔬 6. Hematological Disorders
PAS staining is also applied in hematology to detect abnormal cells:
- Acute Lymphoblastic Leukemia (ALL):
- PAS highlights cytoplasmic glycogen granules in leukemic blasts.
- The characteristic block-like PAS-positive pattern assists in identifying specific ALL subtypes.
- Erythroleukemia:
- Shows PAS-positive erythroid precursors, supporting diagnostic conclusions.
💡 7. Assessment of Mucopolysaccharidoses (MPS)
Mucopolysaccharidoses (MPS) are genetic disorders characterized by the accumulation of glycosaminoglycans (GAGs):
- PAS staining highlights these PAS-positive deposits in tissues.
- Essential for diagnosing and classifying different MPS types.
🌟 Summary of Key Clinical Applications:
| Application Area | Diagnostic Use of PAS Staining |
|---|---|
| Cancer Pathology | Detects mucins, glycogen, and basement membrane integrity. |
| Infectious Diseases | Identifies fungal organisms and bacteria in Whipple’s disease. |
| Metabolic Disorders | Diagnoses glycogen storage diseases and lysosomal disorders. |
| Liver and Kidney Pathology | Assesses glomerular and hepatocellular structural changes. |
| Muscle Disorders | Highlights glycogen accumulation in myopathies. |
| Hematology | Aids in diagnosing leukemia subtypes with characteristic staining patterns. |
| Genetic Disorders | Detects glycosaminoglycan accumulation in mucopolysaccharidoses. |
Variations and Related Staining Techniques
The Periodic Acid–Schiff (PAS) staining technique has several variations and related staining methods that enhance its diagnostic applications. These techniques provide additional specificity, helping distinguish between different tissue components and offering greater diagnostic clarity in various pathological conditions.
🎯 1. PAS-Diastase (PAS-D) Staining
Purpose:
- Differentiates glycogen from other PAS-positive substances like mucins and basement membranes.
- Useful in diagnosing glycogen storage diseases and identifying glycogen-rich tumors.
How It Works:
- Diastase enzyme digests glycogen before PAS staining.
- If staining disappears after diastase treatment, the substance was glycogen (diastase-sensitive).
- If staining persists, it indicates mucins, basement membranes, or other carbohydrates (diastase-resistant).
Clinical Applications:
- Clear Cell Carcinoma: Confirms the presence of glycogen in tumor cells.
- Glycogen Storage Disorders: Differentiates glycogen accumulation in liver and muscle tissues.
- Hepatic Pathology: Identifies alpha-1 antitrypsin deficiency with diastase-resistant PAS-positive globules.
🟣 2. Alcian Blue-PAS Staining
Purpose:
- Combines Alcian Blue (stains acidic mucins blue) with PAS (stains neutral mucins magenta) to distinguish between different types of mucins.
Clinical Significance:
- Gastrointestinal Pathology: Differentiates between intestinal metaplasia (acidic mucins) and gastric epithelium (neutral mucins).
- Respiratory Tract Tumors: Assists in identifying mucin subtypes in lung adenocarcinomas.
- Colorectal Carcinomas: Differentiates between mucin-producing tumor cells.
Key Diagnostic Insight:
- The dual-color pattern (blue for acidic mucins, magenta for neutral mucins) provides detailed information on mucin composition, crucial for tumor characterization and prognosis.
🔵 3. Best’s Carmine Stain
Purpose:
- Specifically stains glycogen in tissues.
- Offers an alternative to PAS staining when glycogen specificity is required.
Key Features:
- Glycogen appears red in stained tissues.
- Unlike PAS, Best’s Carmine does not stain other carbohydrates, providing greater specificity.
Clinical Applications:
- Glycogen Storage Diseases: Precise identification of glycogen in hepatocytes and myocytes.
- Liver Biopsies: Detects glycogen accumulation in metabolic disorders.
🟡 4. Mucicarmine Stain
Purpose:
- Selectively stains epithelial mucins pink to red, providing a more focused identification of mucin-producing cells.
Clinical Importance:
- Adenocarcinomas: Detects mucin production in tumors from the lung, gastrointestinal tract, and breast.
- Cryptococcus neoformans: The capsule of this fungal pathogen stains positively, aiding in its identification.
- Distinguishing Tumors: Differentiates adenocarcinomas from squamous cell carcinomas, as the latter typically lacks mucin.
🧪 5. Grocott’s Methenamine Silver (GMS) Stain
Purpose:
- While primarily used for fungal detection, GMS is often complementary to PAS in infectious disease pathology.
Key Differences from PAS:
- GMS stains fungal elements black, while PAS stains them magenta.
- GMS offers higher contrast in fungal detection, especially in tissues with background staining interference.
Clinical Applications:
- Fungal Infections: Differentiates fungal organisms more distinctly than PAS in some contexts.
- Pulmonary Pathology: Identifies Pneumocystis jirovecii in immunocompromised patients.
🟩 6. Congo Red Stain
Purpose:
- Detects amyloid deposits in tissues, showing apple-green birefringence under polarized light.
- Although unrelated to carbohydrates, Congo Red is often used alongside PAS to exclude amyloidosis when carbohydrate accumulation is suspected.
Clinical Relevance:
- Amyloidosis Diagnosis: Critical in identifying systemic and localized amyloidosis, especially in renal and cardiac biopsies.
- Differential Diagnosis: Helps differentiate amyloid deposits from PAS-positive glycogen or mucin accumulations.
🧬 7. Periodic Acid–Methenamine Silver (PAMS) Stain
Purpose:
- A silver-based variation of PAS used for basement membrane visualization, particularly in renal pathology.
Advantages Over PAS:
- Sharper delineation of glomerular basement membranes.
- Highlights splitting or duplication of basement membranes, crucial for diagnosing conditions like Alport syndrome and membranous glomerulonephritis.
🏃 8. Sudan Stains (Sudan III, IV, and Black)
Purpose:
- Stains lipids, which PAS does not highlight.
- Often used alongside PAS in metabolic disorder diagnosis to differentiate lipid from carbohydrate accumulations.
Clinical Context:
- Fatty Liver Disease: Differentiates steatosis (lipid accumulation) from glycogen storage (PAS-positive).
- Lipid Storage Disorders: Identifies lipid accumulation in metabolic syndromes.
🌟 Summary of PAS Variations and Related Stains:
| Staining Technique | Primary Target | Key Applications | Unique Feature |
|---|---|---|---|
| PAS-Diastase (PAS-D) | Glycogen | Glycogen storage diseases, glycogen-rich tumors | Confirms glycogen presence |
| Alcian Blue-PAS | Acidic & Neutral Mucins | Gastrointestinal metaplasia, lung cancers | Dual staining (blue & magenta) |
| Best’s Carmine | Glycogen | Glycogen storage disorders, liver pathology | High glycogen specificity |
| Mucicarmine | Epithelial mucins | Adenocarcinomas, fungal capsule detection | Selective mucin staining |
| Grocott’s Methenamine Silver (GMS) | Fungal elements | Fungal infections, pulmonary pathology | High-contrast fungal visualization |
| Congo Red | Amyloid | Amyloidosis detection, systemic diseases | Apple-green birefringence |
| PAMS (Silver-PAS) | Basement membranes | Renal pathology (glomerulonephritis, Alport) | Precise basement membrane detail |
| Sudan Stains | Lipids | Fatty liver disease, lipid storage disorders | Differentiates lipids from glycogen |
Troubleshooting Common Issues in PAS Staining
Periodic Acid–Schiff (PAS) staining is a powerful technique, but technical issues can compromise the quality of results. Effective troubleshooting ensures accurate interpretation and reliable diagnostics. This section outlines the common challenges encountered during PAS staining, their underlying causes, and practical solutions to overcome them.
⚡ 1. Weak or No Staining
Possible Causes:
- Expired reagents (especially Schiff’s reagent).
- Insufficient oxidation with periodic acid.
- Over-rinsing after Schiff’s reagent application.
- Tissue over-fixation (particularly with strong fixatives).
Solutions:
- ✅ Check reagent freshness and prepare fresh Schiff’s reagent if needed.
- ✅ Verify oxidation time: Ensure proper exposure to periodic acid (5–10 minutes).
- ✅ Shorten rinsing times post-Schiff reaction to prevent color loss.
- ✅ Optimize fixation: Use 10% neutral buffered formalin and avoid over-fixation.
🟣 2. Non-Specific Background Staining
Possible Causes:
- Inadequate washing between staining steps.
- Over-oxidation of tissues.
- Tissue contamination or improper handling.
Solutions:
- ✅ Increase wash durations between staining steps to remove excess reagents.
- ✅ Reduce oxidation time with periodic acid if over-staining persists.
- ✅ Handle tissues carefully to avoid contamination and artifacts.
🧪 3. Overstaining of Tissue Components
Possible Causes:
- Prolonged exposure to Schiff’s reagent.
- High concentration of periodic acid.
- Improper reagent storage leading to increased potency.
Solutions:
- ✅ Follow recommended exposure times for Schiff’s reagent (10–15 minutes).
- ✅ Dilute periodic acid if over-oxidation is suspected.
- ✅ Store reagents properly at recommended temperatures to maintain stability.
🟡 4. Patchy or Uneven Staining
Possible Causes:
- Incomplete reagent coverage during staining.
- Uneven tissue thickness during sectioning.
- Drying of tissue sections during the staining process.
Solutions:
- ✅ Ensure complete reagent coverage using gentle agitation.
- ✅ Use microtome settings to cut sections at consistent thickness (4–6 µm).
- ✅ Keep slides moist during staining to prevent drying artifacts.
🔵 5. Fading or Loss of Staining After Completion
Possible Causes:
- Excessive washing after Schiff’s reagent.
- Inadequate fixation leading to unstable staining.
- Exposure to strong light or improper mounting media.
Solutions:
- ✅ Limit washing post-Schiff staining to prevent color loss.
- ✅ Use high-quality mounting media and store slides in dark conditions.
- ✅ Ensure complete fixation before starting the staining process.
🟩 6. False Positive Results
Possible Causes:
- Contaminants mimicking PAS positivity (e.g., dust, fungal spores).
- Endogenous enzyme activity not neutralized during preparation.
Solutions:
- ✅ Maintain a clean workspace and use sterile tools.
- ✅ Pre-treat sections with appropriate enzyme inhibitors if necessary.
- ✅ Include proper controls to confirm true positivity.
🧬 7. Inconsistent Results Between Batches
Possible Causes:
- Variation in reagent preparation or batch inconsistencies.
- Differences in incubation times or temperature fluctuations.
Solutions:
- ✅ Standardize protocols with detailed SOPs for each staining batch.
- ✅ Use calibrated timers and controlled environments during staining.
- ✅ Run internal controls with every batch for consistency checks.
🏃 8. Poor Differentiation in PAS-Diastase (PAS-D) Staining
Possible Causes:
- Insufficient diastase digestion leading to incomplete glycogen removal.
- Over-digestion destroying tissue integrity.
Solutions:
- ✅ Optimize diastase digestion times based on tissue type (20–30 minutes at 37°C is standard).
- ✅ Monitor tissue morphology closely to prevent over-digestion.
- ✅ Use appropriate controls to validate glycogen removal.
💡 Pro Tips for Optimal PAS Staining Results:
- 🧼 Clean glassware and slides thoroughly to prevent background staining.
- 🔬 Validate each batch of Schiff’s reagent using known PAS-positive controls.
- 🌡️ Maintain stable temperatures during staining for consistent results.
- 📝 Document any protocol deviations for future reference and troubleshooting.
- 🧪 Use positive and negative controls in each run to ensure staining specificity.
Frequently Asked Questions (FAQs)
❓ What tissues are PAS positive?
Answer:
PAS staining highlights tissues rich in polysaccharides, mucopolysaccharides, and glycoproteins. Common PAS-positive tissues include:
- Liver (due to glycogen storage)
- Kidney tubules (highlighting basement membranes)
- Intestinal epithelium (goblet cells rich in mucins)
- Skin (detecting fungal elements in dermatological biopsies)
- Lung tissue (for mucin detection in respiratory epithelium)
- Cardiac muscle (glycogen-rich areas)
These tissues stain magenta or pink with PAS, indicating the presence of carbohydrate-rich structures.
❓ What does a positive PAS stain indicate?
Answer:
A positive PAS stain indicates the presence of:
- Glycogen: Suggestive of glycogen storage diseases (e.g., von Gierke disease).
- Mucins and glycoproteins: Seen in adenocarcinomas, where mucin production is high.
- Basement membranes: Useful in diagnosing glomerular diseases such as diabetic nephropathy.
- Fungal organisms: Candida, Aspergillus, and Histoplasma have PAS-positive cell walls.
- Certain pathogens: Whipple’s disease shows PAS-positive macrophages in intestinal biopsies.
Thus, positive PAS staining plays a crucial role in diagnosing metabolic disorders, tumors, and infections.
❓ How is PAS staining used in fungal infection diagnosis?
Answer:
PAS staining is a highly sensitive technique for identifying fungal infections because fungal cell walls contain polysaccharides that react with the PAS reagent, staining them bright magenta.
Key applications include:
- Diagnosing Candida albicans infections in esophageal biopsies.
- Identifying Aspergillus in lung tissue.
- Detecting Histoplasma capsulatum in bone marrow or lung samples.
- Differentiating fungal infections from bacterial or viral causes in tissue sections.
The contrast provided by PAS staining helps pathologists visualize fungi even in low concentrations, making it a valuable diagnostic tool.
❓ Is PAS staining specific for glycogen?
Answer:
No, PAS staining is not specific for glycogen. It highlights a broad range of carbohydrate-containing molecules, including:
- Glycoproteins
- Mucopolysaccharides
- Basement membranes
- Fungal polysaccharides
To specifically confirm glycogen, a PAS-Diastase (PAS-D) stain is performed. Diastase enzymatically digests glycogen before staining. If the magenta color disappears after diastase treatment, the positive signal in the PAS stain is confirmed to be glycogen. If the staining persists, other PAS-positive components like mucins or glycoproteins are present.
Conclusion
In this blog post, we’ve explored the fundamentals of PAS staining, from its scientific principles and step-by-step protocol to its applications in pathology and troubleshooting common issues. PAS staining remains an invaluable tool for detecting glycogen, mucins, fungal infections, and more, playing a critical role in diagnostics. With the right technique and attention to detail, PAS staining can provide clear, reliable insights for a wide range of medical and research applications. We hope this guide helps you refine your staining techniques and navigate any challenges with confidence!