Mitochondria are small, membrane-bound organelles found in the cytoplasm of nearly all eukaryotic cells. Often referred to as the “powerhouse of the cell,” mitochondria play a crucial role in producing the energy that cells need to function. Through a process known as cellular respiration, they convert nutrients like glucose and oxygen into adenosine triphosphate (ATP) — the energy currency of the cell.
But mitochondria are more than just energy producers. They are involved in essential cellular processes such as apoptosis (programmed cell death), calcium regulation, and the generation of reactive oxygen species (ROS). Their unique characteristics, including having their own DNA (mitochondrial DNA or mtDNA), set them apart from other organelles and point to their fascinating evolutionary origin through the endosymbiotic theory.
In this article, we’ll explore the structure, functions, genetic features, and clinical significance of mitochondria, uncovering why these tiny organelles are central to life and health.
2. Structure of Mitochondria
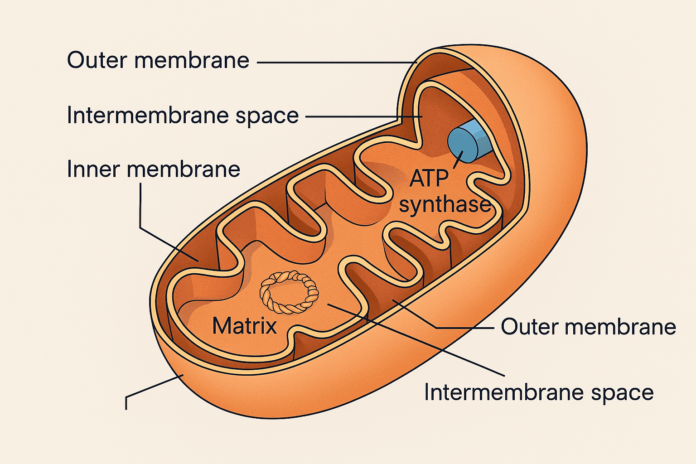
Mitochondria have a double membrane structure that gives them a unique architecture, allowing them to efficiently carry out energy production and other vital cellular functions. Each mitochondrion consists of two membranes — an outer membrane and a highly folded inner membrane — creating distinct internal compartments with specialized roles.
Outer Membrane
The outer mitochondrial membrane encloses the entire organelle and acts as a barrier between the mitochondrion and the cytoplasm. It contains porins, which are protein channels that allow the passage of ions, nutrients, and small proteins.
Inner Membrane
The inner membrane is where the magic of ATP production happens. It is highly folded into structures called cristae, which increase the surface area for the electron transport chain and oxidative phosphorylation. This membrane is selectively permeable and rich in proteins involved in ATP synthesis.
Cristae
Cristae are the inner folds of the mitochondrial inner membrane. These folds maximize surface area, enhancing the mitochondrion’s ability to produce energy. The location of the ATP synthase enzyme and various electron transport proteins makes the cristae critical for energy metabolism.
Intermembrane Space
The intermembrane space lies between the outer and inner membranes. It plays a key role in the proton gradient established during the electron transport chain — essential for ATP production.
Matrix
The mitochondrial matrix is the innermost compartment, enclosed by the inner membrane. It contains mitochondrial DNA (mtDNA), ribosomes, enzymes for the Krebs cycle (citric acid cycle), and other components involved in metabolism and mitochondrial replication.
Summary of Mitochondrial Structure
| Component | Function |
|---|---|
| Outer Membrane | Regulates passage of materials |
| Inner Membrane | Site of ATP production via electron transport |
| Cristae | Increase surface area for energy generation |
| Intermembrane Space | Involved in proton gradient for ATP synthesis |
| Matrix | Contains mtDNA and enzymes for the Krebs cycle |
This complex yet efficient structure allows mitochondria to function as the cell’s main energy producers while also participating in signaling, differentiation, and cell death pathways.
3. Function of Mitochondria
The primary function of mitochondria is to produce energy for the cell. This energy is stored in the form of adenosine triphosphate (ATP), the universal fuel used in nearly all cellular activities. However, mitochondria are involved in much more than just energy metabolism. They play central roles in cellular respiration, apoptosis, calcium regulation, and reactive oxygen species (ROS) production.
🔋 ATP Production through Cellular Respiration
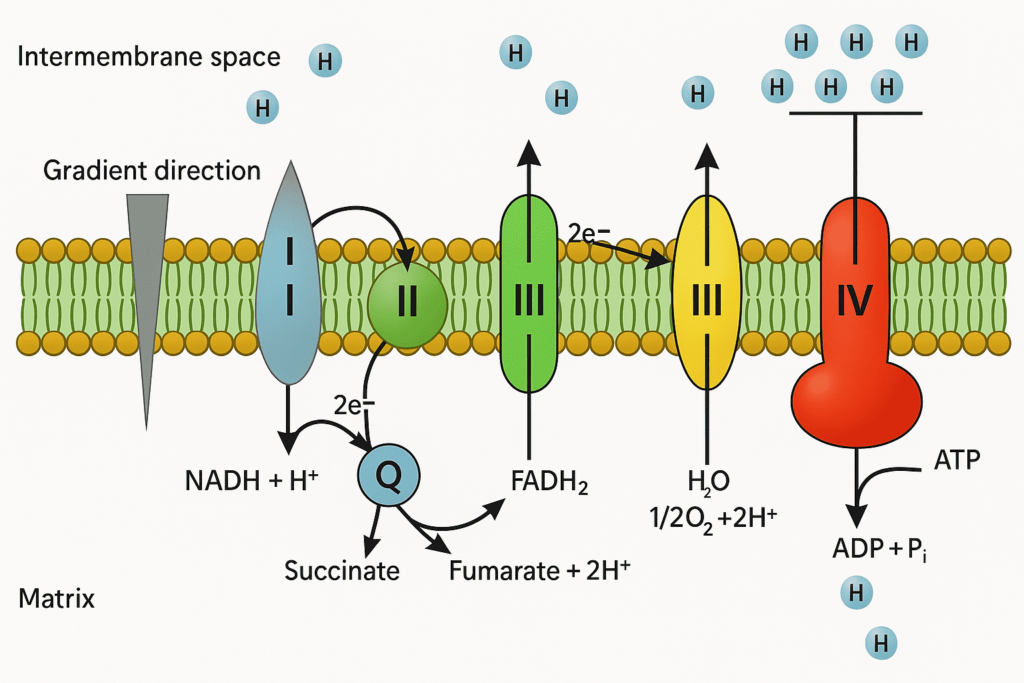
Mitochondria generate ATP through a multi-step process known as aerobic cellular respiration, which involves:
- Glycolysis (in the cytoplasm)
- Krebs cycle (citric acid cycle) — occurs in the mitochondrial matrix
- Electron Transport Chain (ETC) — located on the inner mitochondrial membrane
- Oxidative phosphorylation — final step where ATP is produced using the proton gradient
During this process, electrons from NADH and FADH₂ are passed along a chain of proteins in the inner membrane, ultimately driving the synthesis of ATP by ATP synthase. This mechanism is highly efficient and is the reason mitochondria are referred to as the powerhouse of the cell.
🧪 Other Key Functions of Mitochondria
1. Regulation of Apoptosis (Programmed Cell Death)
Mitochondria control intrinsic apoptosis by releasing molecules such as cytochrome c, which activate caspases — the enzymes that dismantle the cell. This process is essential for eliminating damaged or unneeded cells, and its dysregulation is linked to cancer and neurodegenerative diseases.
2. Calcium Homeostasis
Mitochondria act as reservoirs for calcium ions (Ca²⁺), helping maintain cellular calcium balance. They interact with the endoplasmic reticulum (ER) to regulate calcium signaling pathways crucial for muscle contraction, hormone release, and metabolism.
3. Generation of Reactive Oxygen Species (ROS)
As a byproduct of ATP production, mitochondria produce small amounts of reactive oxygen species, which play roles in cell signaling. However, excess ROS can lead to oxidative stress, damaging proteins, DNA, and lipids — a contributing factor in aging and disease.
4. Metabolism of Amino Acids and Fatty Acids
Mitochondria are involved in the beta-oxidation of fatty acids and the metabolism of amino acids, providing substrates for the Krebs cycle and ATP production.
⚠️ Dysfunction and Disease
When mitochondrial function is impaired, it can lead to a wide range of conditions, including:
- Mitochondrial myopathies
- Leigh syndrome
- Neurodegenerative diseases (e.g., Parkinson’s, Alzheimer’s)
- Cancer and metabolic disorders
🧠 Quick Recap
| Function | Description |
|---|---|
| ATP production | Through oxidative phosphorylation and the ETC |
| Apoptosis regulation | Initiates cell death via cytochrome c and caspases |
| Calcium storage | Regulates intracellular calcium signaling |
| ROS production | Produces reactive oxygen species during respiration |
| Fat & amino acid metabolism | Breaks down nutrients for energy and biosynthesis |
4. Mitochondrial DNA and Inheritance
Mitochondria possess their own genetic material known as mitochondrial DNA (mtDNA), which is distinct from the nuclear DNA found in the cell’s nucleus. Unlike nuclear DNA, mtDNA is circular and encodes genes essential for the organelle’s function, particularly those involved in the electron transport chain and oxidative phosphorylation.
🔍 Key Characteristics of mtDNA
- Circular Genome:
Mitochondrial DNA is typically a small, circular molecule. Its compact form allows it to efficiently encode a subset of proteins, tRNAs, and rRNAs that are critical for mitochondrial energy production. - High Copy Number:
Each mitochondrion contains multiple copies of mtDNA, and cells can harbor hundreds to thousands of mitochondria, ensuring that even if some copies are damaged, others remain functional. - Limited DNA Repair Mechanisms:
mtDNA is more susceptible to damage due to a limited capacity for repair compared to nuclear DNA. This vulnerability means that mutations can accumulate over time, contributing to cellular aging and certain mitochondrial disorders.
👩👧 Maternal Inheritance
One of the most unique aspects of mitochondrial genetics is its maternal inheritance. Unlike nuclear DNA, which is inherited from both parents, mtDNA is passed down almost exclusively from the mother. Here’s why this is significant:
- Exclusivity:
When an egg is fertilized by sperm, the sperm’s mitochondria are typically not transmitted to the embryo. This mechanism ensures that all the mitochondria—and therefore the mtDNA—in a new individual come solely from the mother. - Clinical Implications:
Because of maternal inheritance, mutations in mtDNA can lead to hereditary conditions that are passed from mother to children. Disorders such as Leber’s Hereditary Optic Neuropathy (LHON) and MELAS syndrome are examples of diseases linked to mutations in mtDNA. - Heteroplasmy:
Within a single cell, there can be a mix of both normal and mutated mtDNA, a condition known as heteroplasmy. The proportion of mutated mtDNA can vary among cells, influencing the severity and onset of mitochondrial diseases.
🔗 The Significance of mtDNA in Research
Studying mitochondrial DNA has deepened our understanding of various fields:
- Evolutionary Biology:
Since mtDNA mutates at a relatively constant rate, it serves as a molecular clock to trace maternal lineage and human migration patterns. - Disease Pathology:
Research into mtDNA mutations helps unravel the genetic basis of metabolic and degenerative diseases, emphasizing the importance of mitochondrial health in overall cellular function. - Aging and Therapeutic Approaches:
The accumulation of mtDNA mutations has been linked to the aging process and associated disorders, making it a vital target for potential therapies aimed at improving cellular longevity.
🔑 Quick Recap
| Aspect | Details |
|---|---|
| Type of DNA | Circular, high-copy number, limited repair capabilities |
| Inheritance Pattern | Exclusively maternal, ensuring uniformity in mitochondrial ancestry |
| Clinical Impact | Mutations can lead to conditions like MELAS, LHON, and other disorders |
| Research Importance | Used in studies on evolution, aging, and mitochondrial disease |
Understanding mitochondrial DNA and its mode of inheritance provides valuable insight into both fundamental biology and clinical applications. It not only emphasizes the unique nature of these organelles but also highlights their critical role in human health and disease.
5. Mitochondria and Disease
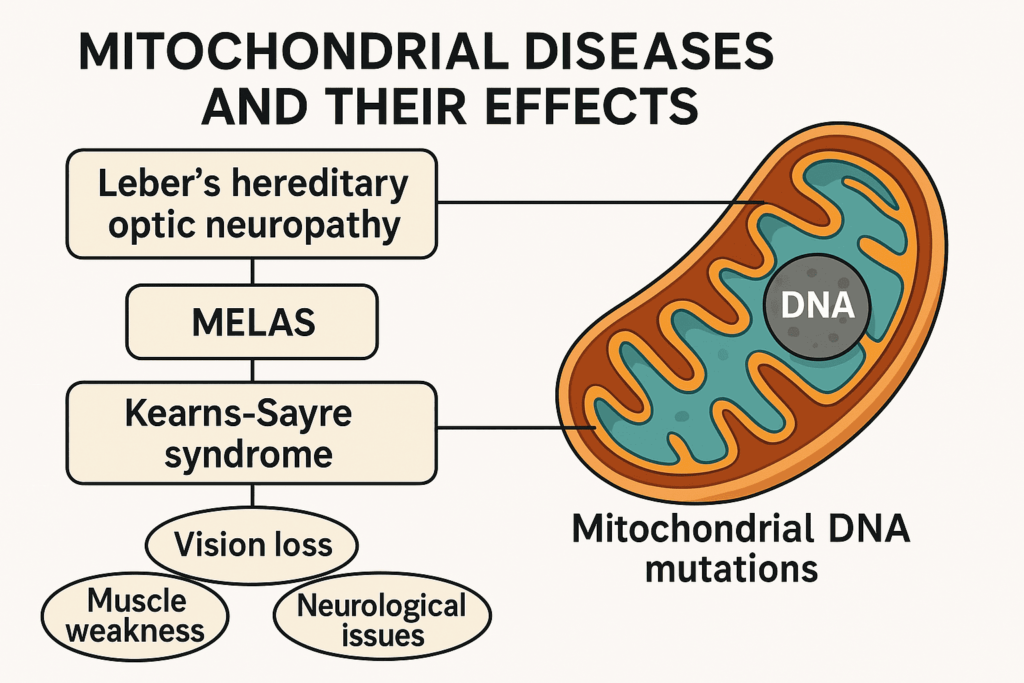
Mitochondria play a central role in maintaining cellular health. When mitochondrial function is compromised, it can lead to a wide range of diseases, from metabolic disorders to neurodegeneration and cancer. These disorders may result from mutations in mitochondrial DNA (mtDNA), defects in nuclear genes that regulate mitochondrial function, or environmental stress that damages mitochondrial components.
1. Mitochondrial Disorders
Mitochondrial diseases are a group of conditions caused by dysfunctional mitochondria. They often affect organs with high energy demands such as the brain, muscles, and heart.
Common mitochondrial disorders include:
- Leber’s Hereditary Optic Neuropathy (LHON):
Leads to sudden vision loss in young adults due to mtDNA mutations. - MELAS Syndrome (Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like episodes):
Affects the nervous system and muscles; caused by mtDNA mutations. - Leigh Syndrome:
A severe neurological disorder that typically appears in infancy or early childhood.
These diseases can be maternally inherited or arise spontaneously, and they often present with complex, multi-system symptoms.
🧠 2. Mitochondria and Neurodegenerative Diseases
Neurons are highly energy-dependent, making them particularly vulnerable to mitochondrial dysfunction.
- Parkinson’s Disease:
Linked to impaired mitochondrial function and increased production of reactive oxygen species (ROS) that damage neuronal cells. - Alzheimer’s Disease:
Associated with mitochondrial abnormalities, oxidative stress, and reduced ATP production in brain cells. - Amyotrophic Lateral Sclerosis (ALS):
Involves mitochondrial damage that contributes to motor neuron degeneration.
🧪 3. Mitochondria and Cancer
Mitochondria play dual roles in cancer: they contribute to tumor growth and survival, but also have the potential to trigger cell death.
- Warburg Effect:
Cancer cells often rely on aerobic glycolysis rather than mitochondrial oxidative phosphorylation for energy — a phenomenon known as the Warburg effect. - Mitochondrial Mutations in Cancer:
Mutations in mtDNA or genes regulating mitochondrial function can promote tumorigenesis, metastasis, and resistance to apoptosis. - Targeted Therapies:
Some anti-cancer strategies aim to disrupt mitochondrial metabolism or restore normal apoptosis pathways in tumor cells.
🔁 4. Mitochondrial Dysfunction and Aging
As we age, mitochondria accumulate mutations, produce more ROS, and lose efficiency in generating ATP. This contributes to:
- Cellular senescence
- Tissue degeneration
- Age-related diseases such as cardiovascular diseases and type 2 diabetes
Many researchers consider mitochondrial dysfunction one of the hallmarks of aging.
🩺 5. Diagnostic and Therapeutic Approaches
- Biomarkers:
mtDNA mutations and changes in mitochondrial enzyme activity are being explored as diagnostic tools. - Gene Therapy and Replacement:
Experimental treatments aim to repair or replace damaged mtDNA, though challenges remain. - Mitochondria-targeted antioxidants (e.g., MitoQ):
Developed to reduce oxidative stress and improve mitochondrial function in chronic diseases.
📌 Summary Table
| Disease Type | Mitochondrial Link |
|---|---|
| Genetic Disorders | Inherited mtDNA mutations (e.g., MELAS, LHON) |
| Neurodegenerative | Mitochondrial dysfunction & ROS in brain cells |
| Cancer | Altered metabolism, mtDNA mutations, resistance to apoptosis |
| Aging-related Diseases | Accumulated mtDNA damage, decreased energy production |
Mitochondria are at the crossroads of health and disease. Their dysfunction can disrupt energy balance, trigger cell death, and contribute to the onset of complex disorders. Understanding these links opens the door to innovative diagnostic tools and mitochondria-targeted therapies that could transform modern medicine.
6. Mitochondria and Apoptosis
Beyond their role in energy production, mitochondria are central regulators of apoptosis, or programmed cell death — a controlled process by which cells self-destruct when they are damaged, infected, or no longer needed. This mitochondrial pathway of apoptosis is crucial for maintaining tissue homeostasis, preventing tumor growth, and defending against viral infections.
🔬 The Intrinsic Pathway of Apoptosis
Apoptosis can be triggered by intrinsic signals (from inside the cell) or extrinsic signals (from outside the cell). Mitochondria are key players in the intrinsic pathway, also called the mitochondrial pathway.
Here’s how it works:
- Cellular Stress or Damage (e.g., DNA damage, oxidative stress, oncogene activation) activates pro-apoptotic proteins from the Bcl-2 family, such as Bax and Bak.
- These proteins induce mitochondrial outer membrane permeabilization (MOMP) — a critical step that allows the release of cytochrome c into the cytosol.
- Cytochrome c binds with Apaf-1 (apoptotic protease activating factor 1) and pro-caspase-9 to form the apoptosome.
- The apoptosome activates caspase-9, which in turn activates executioner caspases like caspase-3 and caspase-7, leading to controlled cellular dismantling.
🧬 Key Molecules Involved
| Molecule | Function |
|---|---|
| Cytochrome c | Released from mitochondria; initiates apoptosome formation |
| Bax/Bak | Promote mitochondrial membrane permeabilization |
| Bcl-2/Bcl-xL | Anti-apoptotic proteins; inhibit Bax/Bak activity |
| Caspases | Proteolytic enzymes that execute the apoptotic program |
| Apaf-1 | Binds cytochrome c to form the apoptosome |
🔁 Mitochondria: A Double-Edged Sword
Mitochondria both sustain life through energy production and trigger cell death through apoptosis. This balance is essential. When apoptosis fails, cancer cells survive and proliferate. Conversely, excessive apoptosis can contribute to neurodegeneration and tissue damage.
🧠 Clinical Implications
- Cancer:
Many cancers show dysregulation of apoptotic pathways, often through overexpression of anti-apoptotic proteins like Bcl-2. Targeting these proteins can restore apoptosis in tumor cells — an approach used in therapies like Venetoclax, a Bcl-2 inhibitor. - Neurodegenerative Diseases:
Diseases like Parkinson’s and Alzheimer’s involve excessive mitochondrial-mediated apoptosis, leading to neuron loss. - Autoimmune Disorders:
Impaired apoptosis can allow autoreactive immune cells to persist, contributing to diseases like lupus.
🧪 Therapeutic Research Directions
Scientists are exploring ways to:
- Modulate Bcl-2 family proteins to control apoptosis
- Target mitochondrial membranes to induce apoptosis in cancer cells
- Use small molecules that selectively trigger apoptosis in diseased cells
✅ Quick Summary
- Mitochondria regulate intrinsic apoptosis by releasing cytochrome c.
- A cascade of protein interactions leads to the activation of caspases and cell death.
- Dysregulation of mitochondrial apoptosis contributes to cancer, neurodegeneration, and autoimmune diseases.
- Targeting mitochondrial apoptosis pathways offers promising therapeutic strategies.
Understanding how mitochondria orchestrate cell death reveals just how vital they are—not only in sustaining life but in eliminating threats to cellular health. The fine-tuned balance they maintain is a cornerstone of modern biomedical research.
🔄 7. Mitochondrial Biogenesis and Dynamics
Mitochondria are dynamic organelles that constantly adapt to cellular needs through processes of biogenesis, fission, fusion, and mitophagy. These mechanisms are crucial for maintaining mitochondrial health, ensuring efficient energy production, and responding to environmental and metabolic changes.
⚙️ Mitochondrial Biogenesis: Making New Mitochondria
The cell forms new mitochondria through the process of mitochondrial biogenesis. This involves:
- Replication of mitochondrial DNA (mtDNA)
- Synthesis of mitochondrial proteins
- Import of nuclear-encoded proteins
- Assembly of mitochondrial membranes and complexes
Biogenesis is primarily regulated by the PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha), which activates transcription factors like NRF1, NRF2, and TFAM (mitochondrial transcription factor A). These factors drive the expression of genes involved in mitochondrial replication and function.
🔬 When Does Biogenesis Increase?
- During exercise
- In response to cold exposure
- Following caloric restriction
- As an adaptation to oxidative stress or injury
🔁 Mitochondrial Dynamics: Fission and Fusion
Mitochondria are not static; they are constantly reshaped through two opposing processes:
🔹 1. Mitochondrial Fusion
- Purpose: Combines mitochondria to mix contents, dilute damage, and support function.
- Key Proteins: Mitofusins (MFN1, MFN2) on the outer membrane and OPA1 on the inner membrane.
- Benefits: Promotes mitochondrial complementation, energy efficiency, and cellular survival.
🔸 2. Mitochondrial Fission
- Purpose: Splits mitochondria, allowing damaged parts to be isolated and removed.
- Key Protein: DRP1 (Dynamin-related protein 1)
- Benefits: Facilitates cell division, apoptosis, and removal of dysfunctional mitochondria via mitophagy.
A balanced cycle of fusion and fission is essential for:
- Cellular differentiation
- Adaptation to metabolic demands
- Response to stress and damage
🗑️ Mitophagy: Quality Control
Damaged or dysfunctional mitochondria are selectively removed through mitophagy, a specialized form of autophagy. This process prevents the accumulation of defective mitochondria that could lead to oxidative stress, inflammation, or cell death.
- Key Regulators: PINK1 and Parkin
- Importance: Especially critical in long-lived cells like neurons and muscle fibers
🔬 Clinical Relevance
- Neurodegenerative Diseases: Impaired mitochondrial dynamics are implicated in Parkinson’s, Alzheimer’s, and Huntington’s diseases.
- Cancer: Altered mitochondrial fusion and fission help tumor cells adapt to hypoxia, resist apoptosis, and maintain metabolic flexibility.
- Metabolic Disorders: Defects in biogenesis and mitophagy are linked to insulin resistance and type 2 diabetes.
🧠 Summary Table
| Process | Function | Key Proteins |
|---|---|---|
| Biogenesis | Creation of new mitochondria | PGC-1α, NRF1, TFAM |
| Fusion | Joins mitochondria for functional support | MFN1, MFN2, OPA1 |
| Fission | Splits mitochondria for division or removal | DRP1 |
| Mitophagy | Degrades damaged mitochondria | PINK1, Parkin |
Maintaining a dynamic mitochondrial network through biogenesis, fusion, fission, and mitophagy is essential for cellular energy balance, adaptation, and survival. Disruptions in these processes are increasingly recognized as key contributors to aging and disease — making them exciting targets for therapeutic intervention.
8. The Endosymbiotic Theory
One of the most fascinating aspects of mitochondria is their evolutionary origin. The endosymbiotic theory proposes that mitochondria originated from a free-living prokaryote—likely a type of alpha-proteobacterium—that was engulfed by a primitive eukaryotic cell around 1.5 to 2 billion years ago.
🧫 What Is the Endosymbiotic Theory?
According to this theory:
- An ancestral aerobic bacterium entered a larger host cell (an early eukaryote).
- The host did not digest the bacterium but instead formed a mutualistic relationship with it.
- Over time, the bacterium became a permanent organelle, evolving into what we now recognize as the mitochondrion.
This symbiotic event gave eukaryotic cells the ability to produce ATP efficiently through aerobic respiration, allowing the rise of more complex life forms.
🔍 Evidence Supporting the Endosymbiotic Theory
Multiple lines of evidence support the theory that mitochondria evolved from prokaryotic ancestors:
| Feature | Mitochondria | Bacteria |
|---|---|---|
| Double membrane | Present | Present in gram-negative bacteria |
| Circular DNA | Like bacterial DNA | Circular chromosome |
| 70S ribosomes | Same type as in bacteria | Found in prokaryotes |
| Binary fission | Replicate independently like bacteria | Typical prokaryotic division |
| Gene similarity | mtDNA is similar to alpha-proteobacterial genes | Supports a shared ancestor |
🌱 Why Is This Theory Important?
Understanding the origin of mitochondria through the endosymbiotic theory has far-reaching implications:
- It highlights how eukaryotic complexity evolved from simpler prokaryotic ancestors.
- It explains why mitochondrial diseases are maternally inherited (mitochondria come from the mother’s egg).
- It helps us grasp how horizontal gene transfer and symbiosis have shaped evolution.
🧠 Fun Fact
Some scientists suggest that chloroplasts in plant cells also evolved via a similar endosymbiotic event — but with photosynthetic cyanobacteria instead.
✅ Quick Takeaway
The endosymbiotic theory explains that mitochondria were once independent bacteria that took up residence inside a host cell, giving rise to the powerful, energy-producing organelles we see today. This evolutionary partnership was a key moment in the development of complex life on Earth.
9. Mitochondria in Different Cell Types
Mitochondria are not a one-size-fits-all organelle. Their function, structure, and distribution can vary greatly across different types of cells. This variation reflects the specific energy demands and roles of each cell type in the body. Let’s explore how mitochondria adapt to the unique needs of various cell types.
💪 Mitochondria in Muscle Cells
Muscle cells, especially skeletal muscle fibers and cardiomyocytes (heart muscle cells), have a high density of mitochondria due to their massive energy requirements. These cells rely heavily on oxidative phosphorylation for ATP production, particularly during sustained activity or exercise.
- Skeletal Muscle Cells (Myocytes):
These cells contain two main types of muscle fibers—Type I (slow-twitch) and Type II (fast-twitch)—which differ in mitochondrial content:- Type I fibers have more mitochondria and rely on aerobic respiration for endurance.
- Type II fibers have fewer mitochondria and rely more on anaerobic metabolism for quick, explosive energy bursts.
- Cardiac Muscle Cells (Cardiomyocytes):
These cells have an exceptionally high mitochondrial content (up to 40% of cell volume) to meet the heart’s continuous energy needs for contraction and blood circulation.
🧠 Mitochondria in Brain Cells
The brain is another organ with high energy demands. Neurons (nerve cells) require a constant supply of ATP to maintain membrane potential and neurotransmitter signaling. However, Different types of neurons meet energy demands in different ways.:
- Excitatory Neurons:
These neurons, which release glutamate as a neurotransmitter, rely heavily on mitochondria to fuel the active transport of ions across membranes during action potentials. - Inhibitory Neurons:
These neurons, which release GABA, require energy to maintain synaptic transmission and balance excitatory signaling, a function that also demands a high mitochondrial presence. - Astrocytes:
These glial cells provide metabolic support to neurons and have mitochondria that are more involved in lactate shuttle and energy buffering, ensuring a steady supply of fuel for neurons.
🦠 Mitochondria in Immune Cells
Immune cells, such as T cells, B cells, and macrophages, undergo significant changes in mitochondrial function during immune activation.
- T cells:
Upon activation, T cells increase mitochondrial biogenesis and shift to aerobic glycolysis for rapid ATP production to support cell division and function during immune responses. - Macrophages:
Macrophages adjust mitochondrial activity based on the type of immune response:- During inflammation, macrophages shift to glycolysis for energy production.
- In contrast, during resolution of inflammation, they rely on oxidative phosphorylation for more efficient energy production.
🧬 Mitochondria in Epithelial Cells
Epithelial cells line the surfaces of organs and tissues and vary in their mitochondrial content depending on their function.
- Intestinal Epithelial Cells:
These cells have mitochondria to support their role in nutrient absorption and active transport across the intestinal lining. - Kidney Tubule Cells:
Cells in the proximal tubule of the kidneys have a high mitochondrial density to support active transport of ions and solutes, maintaining fluid and electrolyte balance.
🔬 Mitochondria in Oocytes (Egg Cells)
Mitochondria play a crucial role in oocyte maturation and early embryonic development. The egg cell (oocyte) contains a large number of mitochondria to support the energy demands of fertilization and early cell division.
- Mitochondrial inheritance is maternal, meaning the mitochondria in the zygote come from the mother’s egg. The mother passes down certain mitochondrial diseases through maternal inheritance.
🧬 Mitochondria in Cancer Cells
Cancer cells exhibit unique adaptations in mitochondrial function, primarily driven by their need to sustain rapid growth and survival in harsh environments.
- Many cancer cells rely on the Warburg effect, where they shift to aerobic glycolysis even in the presence of oxygen. However, mitochondria still play a role in maintaining the metabolic flexibility required for tumor progression and metastasis.
- Mitochondrial fusion and fission in cancer cells enable them to adapt to nutrient deprivation and avoid apoptosis, contributing to chemoresistance.
🧪 Summary Table: Mitochondrial Characteristics in Different Cells
| Cell Type | Mitochondrial Role | Key Feature |
|---|---|---|
| Skeletal Muscle | ATP production during exercise | High mitochondrial density, endurance vs. explosive fibers |
| Cardiac Muscle | Continuous ATP production for heart contraction | High mitochondrial content, essential for heart function |
| Neurons | ATP production for membrane potential and signaling | High energy demand for neurotransmission |
| T Cells | Energy for activation and proliferation during immune response | Mitochondrial biogenesis during activation |
| Macrophages | ATP production during immune responses | Switch between glycolysis and oxidative phosphorylation |
| Epithelial Cells | ATP for active transport and absorption | Mitochondria adapt to specialized functions |
| Oocytes | Energy for fertilization and early development | Maternal inheritance, high mitochondrial content |
| Cancer Cells | Metabolic flexibility and resistance to apoptosis | Altered metabolism, mitochondria contribute to survival |
Conclusion
Mitochondria are much more than just the cell’s powerhouses—they are dynamic organelles with critical roles in energy production, cellular regulation, and even disease. From their unique evolutionary origin through the endosymbiotic theory to their diverse functions in various cell types, mitochondria are essential for maintaining cellular health. Understanding their complexity can open new doors for therapeutic strategies in treating mitochondrial diseases, cancer, and other conditions. As research continues to uncover the many secrets of mitochondria, their importance in both health and disease becomes ever more evident.