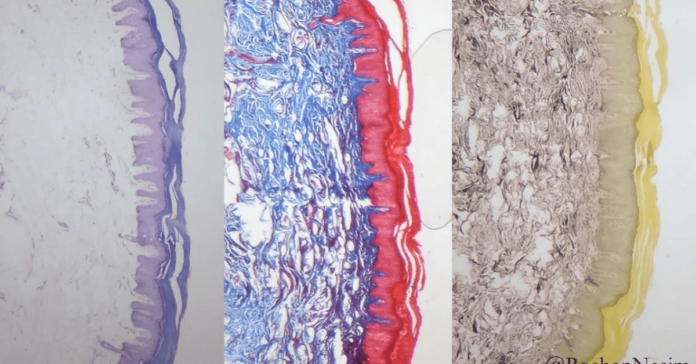
Masson’s Trichrome stain is a widely used histological staining technique that plays a crucial role in differentiating between various tissue components, particularly collagen fibers, muscle tissue, and cytoplasm. By providing distinct color contrasts—typically blue or green for collagen, red for muscle fibers, and dark for nuclei—this stain allows researchers and pathologists to visualize tissue architecture with remarkable clarity.
Originally developed by Claude L. Pierre Masson in the early 20th century, Masson’s Trichrome stain has become an essential tool in histopathological analysis, especially in the study of fibrosis, cancer diagnostics, and connective tissue diseases. Its ability to highlight structural changes in tissues makes it invaluable for identifying pathological conditions such as liver fibrosis, myocardial fibrosis, and tumor invasion patterns in cancer research.
In this article, we will explore the principle, protocol, and interpretation of Masson’s Trichrome staining results. We will also discuss its applications in clinical and research settings, address common troubleshooting tips, and compare it with other histological staining techniques. Whether you are a researcher, student, or healthcare professional, this comprehensive guide will help you understand the significance and practical uses of Masson’s Trichrome stain in modern pathology.
⭐ 2. Principle of Masson’s Trichrome Staining
The principle of Masson’s Trichrome staining lies in its ability to differentiate between various components of tissue based on their chemical composition and affinity for specific dyes. This staining technique uses a combination of three dyes that selectively bind to tissue elements, allowing for clear visualization of collagen fibers, muscle tissue, cytoplasm, and cell nuclei.
🎨 How the Stain Works
The staining process relies on differences in tissue permeability and dye-binding properties:
- Nuclei are stained dark blue or black using hematoxylin, highlighting the cell’s genetic material.
- Muscle fibers, cytoplasm, and keratin take up the Biebrich scarlet-acid fuchsin dye, appearing red.
- Collagen fibers and mucin are stained blue or green depending on the dye used (Aniline blue for blue staining or Light Green SF for green staining).
The result is a distinct color contrast that makes it easy to distinguish between collagen and muscle tissues—an essential feature in detecting fibrosis, scar tissue formation, and assessing tumor invasion in cancer pathology.
🔍 Key Steps in the Staining Mechanism
- Fixation and Mordanting
- Bouin’s solution is commonly used as a mordant to enhance tissue permeability, allowing dyes to penetrate effectively and intensify staining contrast.
- Application of Acid Dyes
- The Biebrich scarlet-acid fuchsin dye stains the cytoplasm, muscle fibers, and keratin. This dye combination binds strongly to these components due to their acidic nature.
- Differentiation with Phosphomolybdic-Phosphotungstic Acid
- This step removes the red dye from collagen fibers, leaving them unstained while muscle tissue remains red. This selective removal is crucial for clear differentiation.
- Collagen Staining
- Aniline blue or Light Green SF is then applied, which binds specifically to the collagen fibers, coloring them blue or green, respectively.
🧬 Why Masson’s Trichrome Stain Is Important
- Collagen detection: Critical in diagnosing fibrotic diseases such as liver cirrhosis, cardiac fibrosis, and kidney fibrosis.
- Cancer diagnostics: Helps assess the tumor stroma and invasion patterns, which are vital in understanding tumor aggressiveness.
- Muscle tissue analysis: Differentiates muscle degeneration from fibrotic replacement in muscular disorders.
🧪 3. Reagents and Materials Required for Masson’s Trichrome Staining
The Masson’s Trichrome staining protocol requires a set of specific reagents and materials to ensure accurate and reproducible results. Each reagent plays a crucial role in highlighting different tissue components, providing the characteristic tricolor appearance that distinguishes collagen, muscle fibers, and nuclei.
🧬 Essential Reagents
- Fixative:
- Bouin’s Solution (picric acid, formaldehyde, and acetic acid)
- Purpose: Acts as a mordant to enhance dye penetration and improve color contrast. It also helps preserve tissue architecture.
- Note: Bouin’s solution can be used as a fixative or as a post-fixation treatment before staining.
- Bouin’s Solution (picric acid, formaldehyde, and acetic acid)
- Nuclear Stain:
- Weigert’s Iron Hematoxylin
- Purpose: Stains cell nuclei dark blue or black, making nuclear details more prominent.
- Why Iron Hematoxylin? It is resistant to acidic solutions used later in the protocol, ensuring nuclei remain stained.
- Weigert’s Iron Hematoxylin
- Cytoplasmic and Muscle Stain:
- Biebrich Scarlet-Acid Fuchsin Solution
- Purpose: Stains cytoplasm, muscle fibers, and keratin red.
- Function: This acidic dye complex binds strongly to tissue components with high protein content.
- Biebrich Scarlet-Acid Fuchsin Solution
- Differentiation Solution:
- Phosphomolybdic-Phosphotungstic Acid
- Purpose: Removes the red dye from collagen fibers while retaining it in muscle and cytoplasm.
- Function: This selective removal ensures clear differentiation between muscle (red) and collagen (blue/green).
- Phosphomolybdic-Phosphotungstic Acid
- Collagen Stain:
- Aniline Blue or Light Green SF Yellowish
- Aniline Blue: Stains collagen fibers blue (commonly used for general histology).
- Light Green SF: Stains collagen fibers green (preferred when greater contrast with red-stained muscle is desired).
- Aniline Blue or Light Green SF Yellowish
- Acetic Acid (1%)
- Purpose: Stabilizes the staining and sharpens color differentiation between tissue components.
🧪 Additional Materials and Equipment
- Tissue sections (formalin-fixed, paraffin-embedded)
- Microtome (for sectioning tissues at 4–5 µm thickness)
- Glass slides and coverslips
- Staining jars and racks
- Water bath (for section stretching)
- Xylene and ethanol series (for deparaffinization and rehydration)
- Distilled water (for washing steps)
- Microscope (for result evaluation and interpretation)
⚡ Optional Reagents (Depending on Protocol Variations):
- Picric acid solution (for enhanced contrast in some protocols)
- Mounting medium (e.g., DPX or Canada balsam for permanent slide preservation)
🔍 Safety Precautions:
- Bouin’s solution contains picric acid, which is explosive when dry. Handle with care and dispose of according to safety regulations.
- Use gloves, lab coats, and protective eyewear when handling dyes and chemicals.
- Work in a fume hood when using volatile solvents like xylene.
🧪 4. Masson’s Trichrome Staining Protocol
The Masson’s Trichrome staining protocol involves a series of well-defined steps designed to differentiate collagen fibers, muscle tissue, and nuclei in histological sections. Following this step-by-step guide ensures clear, reproducible results ideal for diagnostic and research purposes.
⚡ Step 1: Tissue Preparation
- Deparaffinization:
- Immerse slides with paraffin-embedded tissue sections in xylene for 2 × 5 minutes to remove paraffin.
- Rehydration:
- Transfer slides through a descending ethanol series:
- 100% ethanol: 2 × 3 minutes
- 95% ethanol: 2 minutes
- 70% ethanol: 2 minutes
- Rinse slides in distilled water for 5 minutes.
- Transfer slides through a descending ethanol series:
🌡️ Step 2: Bouin’s Solution Treatment (Mordanting)
- Place slides in preheated Bouin’s solution at 56°C for 1 hour or at room temperature overnight.
- Purpose: Enhances tissue permeability and intensifies staining contrast.
- After treatment, cool the slides and wash them under running tap water until the yellow color is removed (approximately 5–10 minutes).
🖋️ Step 3: Nuclear Staining
- Stain slides with Weigert’s Iron Hematoxylin for 10 minutes.
- Function: Stains nuclei black and resists decolorization by subsequent acidic solutions.
- Rinse thoroughly in running tap water for 10 minutes.
🩸 Step 4: Cytoplasm and Muscle Staining
- Stain with Biebrich Scarlet-Acid Fuchsin solution for 10–15 minutes.
- Function: Stains cytoplasm, muscle fibers, and keratin red.
- Rinse slides in distilled water to remove excess stain.
⚖️ Step 5: Differentiation of Collagen and Muscle
- Immerse slides in Phosphomolybdic-Phosphotungstic Acid solution for 10–15 minutes.
- Purpose: Differentiates collagen by removing the red dye from collagen fibers while leaving muscle and cytoplasm stained.
- Without rinsing, proceed immediately to the next step.
🎨 Step 6: Collagen Staining
- Stain with either:
- Aniline Blue (for blue collagen) for 5–10 minutes, or
- Light Green SF Yellowish (for green collagen) for 5–10 minutes.
- Rinse slides briefly in distilled water to remove excess dye.
🧪 Step 7: Final Differentiation and Stabilization
- Treat slides with 1% acetic acid for 2–5 minutes to remove background staining and enhance contrast.
- Rinse thoroughly in distilled water for 5 minutes.
🧼 Step 8: Dehydration, Clearing, and Mounting
- Dehydration:
- Pass slides through an ascending ethanol series (70%, 95%, and 100%) for 2 minutes each.
- Clearing:
- Immerse slides in xylene for 2 × 5 minutes.
- Mounting:
- Mount coverslips using a permanent mounting medium (e.g., DPX).
🔬 Step 9: Microscopic Examination
- Examine stained sections under a light microscope.
- Expected results:
- Collagen fibers: Blue (Aniline Blue) or Green (Light Green SF)
- Muscle fibers & Cytoplasm: Red
- Nuclei: Black
⚡ Key Notes & Troubleshooting Tips:
- Uneven staining: Ensure even heating during Bouin’s solution treatment and thorough washing.
- Weak nuclear staining: Check the freshness of Weigert’s Iron Hematoxylin.
- Faint collagen color: Increase staining time with Aniline Blue or Light Green SF if needed.
- Background staining: Extend treatment with 1% acetic acid for better differentiation.
🔍 5. Interpretation of Staining Results
The interpretation of Masson’s Trichrome staining results is essential for identifying and differentiating various tissue components based on their color. This staining technique highlights structural differences in tissues, making it invaluable for diagnosing conditions involving fibrosis, tumor invasion, and muscle pathology.
🎨 Color Interpretation and Tissue Components
The characteristic tricolor appearance allows easy differentiation of:
| Tissue Component | Color (Aniline Blue Method) | Color (Light Green SF Method) | Significance |
|---|---|---|---|
| Collagen fibers | Blue | Green | Indicates connective tissue; abundance suggests fibrosis or scarring. |
| Muscle fibers | Red | Red | Highlights muscle tissue integrity and abnormalities. |
| Cytoplasm | Red | Red | Shows cell body details; useful in tumor cell identification. |
| Nuclei | Black | Black | Highlights nuclear morphology; crucial for detecting malignancy. |
| Erythrocytes (RBCs) | Yellow/Orange | Yellow/Orange | Confirms vascular structures. |
| Keratin | Red | Red | Identifies epithelial tissues and certain tumors. |
🔬 Diagnostic Implications
- Fibrosis Detection
- Collagen-rich areas (stained blue or green) indicate fibrotic tissue.
- Common in liver cirrhosis, myocardial fibrosis, and pulmonary fibrosis.
- Interpretation Tip: The extent of collagen staining can help grade fibrosis severity.
- Tumor Invasion Assessment
- Invasive tumors often disrupt normal tissue architecture.
- Blue/green collagen fibers surrounding red-stained tumor cells may indicate desmoplastic reactions (fibrous tissue response to tumor invasion).
- Muscle Pathology Analysis
- Red-stained muscle fibers should appear uniform.
- Disorganization, atrophy, or replacement by collagen may suggest muscle degeneration or inflammatory myopathies.
- Vascular Structure Identification
- Blood vessels can be identified by the yellow/orange staining of erythrocytes and surrounding blue/green collagen.
- Helps assess angiogenesis in tumors and vascular diseases.
⚡ Clinical Case Examples
- Liver Biopsy (Cirrhosis):
- Blue collagen fibers surrounding regenerative nodules confirm hepatic fibrosis.
- Breast Carcinoma:
- Red tumor cells infiltrating blue-stained stroma indicate invasive carcinoma.
- Cardiac Muscle Biopsy:
- Blue collagen replacing red muscle fibers suggests myocardial fibrosis post-infarction.
📝 Common Interpretation Challenges & Solutions
| Challenge | Potential Cause | Solution |
|---|---|---|
| Faint collagen staining | Insufficient collagen dye time | Increase aniline blue/light green staining time. |
| Poor nuclear definition | Degraded hematoxylin solution | Use freshly prepared Weigert’s hematoxylin. |
| Background staining | Inadequate acetic acid step | Extend acetic acid treatment to reduce background. |
| Overstained muscle fibers | Excessive acid fuchsin staining | Reduce staining time or increase differentiation step. |
🎯 6. Applications of Masson’s Trichrome Stain
The Masson’s Trichrome stain is a powerful histological tool widely used in pathology and research. Its ability to distinguish collagen fibers, muscle tissue, and cellular components makes it essential for studying cancer progression, tumor microenvironments, and fibrotic diseases.
🧬 A. Applications in Cancer
- Assessment of Tumor Invasion
- Purpose: Evaluates how deeply a tumor invades surrounding tissues.
- How it helps:
- Red-stained tumor cells infiltrating blue/green collagenous stroma signal invasive growth.
- Differentiates between non-invasive and invasive carcinomas (e.g., breast, colorectal, and bladder cancers).
- Example: In bladder cancer, the degree of invasion into the muscularis propria (red muscle fibers) impacts staging and treatment decisions.
- Characterization of the Tumor Microenvironment (TME)
- Purpose: Analyzes stromal components and extracellular matrix (ECM) remodeling in cancer.
- Significance:
- Collagen deposition (blue/green) indicates desmoplastic reactions, often associated with aggressive tumors.
- The density and organization of collagen fibers correlate with tumor stiffness, impacting metastasis.
- Example: In pancreatic ductal adenocarcinoma (PDAC), dense collagen-rich stroma affects drug delivery and immune cell infiltration, influencing prognosis.
- Evaluating Fibrosis Associated with Tumors
- Many tumors induce fibrotic responses that can promote cancer progression.
- Masson’s Trichrome highlights these fibrotic changes, helping distinguish between benign fibrous tissue and malignant desmoplasia.
- Example: In breast cancer, extensive fibrosis around tumor cells can indicate a higher grade tumor.
- Tumor Angiogenesis Analysis
- Identifies blood vessels by staining collagen-rich vessel walls (blue/green) and red blood cells (yellow/orange).
- Importance: Understanding angiogenesis patterns aids in evaluating tumor growth and potential anti-angiogenic therapies.
🌡️ B. Applications in Fibrosis
- Grading and Staging of Fibrotic Diseases
- Fibrosis is characterized by the excessive deposition of collagen fibers.
- Masson’s Trichrome stain enables:
- Visualization of collagen accumulation (blue/green).
- Accurate fibrosis staging, essential for treatment decisions.
- Example:
- In liver cirrhosis, it identifies fibrotic septa and nodular regeneration.
- Ishak or METAVIR scoring systems often rely on trichrome staining to assess fibrosis levels.
- Differentiation Between Fibrosis and Inflammation
- Distinguishes fibrosis (blue/green collagen) from inflammatory infiltrates (red cytoplasm).
- Clinical Relevance: Important in conditions where inflammation may lead to fibrosis, such as chronic hepatitis or pulmonary fibrosis.
- Myocardial Fibrosis Detection
- Detects fibrotic tissue (blue/green) replacing healthy cardiac muscle (red).
- Clinical Insight:
- Post-myocardial infarction, fibrosis affects cardiac function and prognosis.
- Helps assess the effectiveness of anti-fibrotic therapies.
- Pulmonary Fibrosis Evaluation
- Identifies fibrotic remodeling in lung tissue.
- Example: In idiopathic pulmonary fibrosis (IPF), collagen deposition patterns guide diagnosis and treatment decisions.
🔬 C. Research Applications
- Drug Response Studies:
- Used in preclinical models to evaluate how therapies affect ECM remodeling and fibrosis in tumors.
- Stem Cell Research:
- Assesses tissue regeneration by highlighting collagen deposition in regenerative medicine.
- Cancer-Fibrosis Crosstalk:
- Explores how fibrotic stroma influences cancer cell invasion, metastasis, and drug resistance.
⚡ 7. Troubleshooting and Limitations of Masson’s Trichrome Stain
While Masson’s Trichrome stain is a robust and widely used technique for differentiating tissue components, it can present technical challenges and has certain limitations. Understanding these issues is essential for obtaining accurate and reproducible results.
🛠️ A. Troubleshooting Common Issues
| Problem | Possible Cause | Solution |
|---|---|---|
| Weak Collagen Staining | – Insufficient staining with aniline blue or light green- Over-differentiation | – Increase staining time for collagen dye- Reduce differentiation step duration |
| Faint Nuclear Staining | – Old or degraded Weigert’s hematoxylin- Inadequate staining time | – Prepare fresh hematoxylin- Extend nuclear staining duration |
| Overstained Cytoplasm | – Excessive Biebrich scarlet-acid fuchsin staining | – Shorten cytoplasmic staining time- Increase differentiation step to balance staining |
| High Background Staining | – Incomplete washing between staining steps- Inadequate acetic acid treatment | – Ensure thorough rinsing after each step- Increase acetic acid incubation time |
| Non-Specific Staining | – Poor tissue fixation- Incomplete paraffin removal | – Use neutral buffered formalin for fixation- Ensure complete deparaffinization with xylene |
| Inconsistent Staining Results | – Variations in reagent concentration- Uneven reagent application | – Standardize reagent preparation- Use automated stainers for consistency |
🔬 B. Best Practices for Optimal Results
- Standardized Fixation:
- Use 10% neutral buffered formalin for at least 24 hours to prevent tissue degradation.
- Controlled Differentiation Step:
- The use of phosphomolybdic-phosphotungstic acid for differentiation must be carefully timed; over-differentiation can lead to loss of cytoplasmic staining.
- Reagent Quality:
- Use freshly prepared solutions, especially Weigert’s iron hematoxylin, to ensure sharp nuclear staining.
- Section Thickness:
- 4–5 µm tissue sections are ideal; thicker sections can result in uneven staining and obscure tissue details.
- Consistent Protocol Timing:
- Strictly adhere to staining times to minimize variability between slides.
🚫 C. Limitations of Masson’s Trichrome Stain
- Lack of Cellular Specificity
- The stain primarily differentiates tissue structures (collagen, muscle, nuclei) but cannot identify specific cell types (e.g., immune cells or cancer subtypes).
- Solution: Combine with immunohistochemistry (IHC) for precise cellular identification.
- Subjective Interpretation
- Color intensity and pattern interpretation can be subjective, leading to inter-observer variability.
- Solution: Implement digital pathology tools for quantitative analysis of stained slides.
- Limited Sensitivity for Early Fibrosis
- Early fibrotic changes may not be well-detected due to low collagen content, potentially leading to underdiagnosis.
- Solution: Use more sensitive staining techniques like Sirius Red, which provides enhanced collagen visualization under polarized light.
- Inability to Differentiate Collagen Subtypes
- Masson’s Trichrome does not distinguish between collagen type I and III, which may be important in some pathological conditions.
- Solution: Utilize immunohistochemical stains specific for collagen subtypes if required.
- Reagent Variability
- Commercially available kits may differ slightly in composition, affecting staining outcomes.
- Solution: Use consistent reagent sources or custom formulations when reproducibility is critical.
🏥 D. Impact of Limitations in Clinical Settings
- Cancer Diagnosis:
- The inability to distinguish between different collagen subtypes or specific stromal components may limit insights into tumor-stroma interactions critical for cancer prognosis.
- Fibrosis Grading:
- For precise staging of fibrotic diseases, subtle collagen deposition in early stages may be underestimated with Masson’s Trichrome alone.
💡 E. Complementary Techniques
| Alternative Technique | Advantage | Use Case |
|---|---|---|
| Sirius Red Stain | Differentiates collagen I and III under polarized light | Detailed fibrosis analysis |
| Immunohistochemistry (IHC) | Identifies specific proteins and cell markers | Tumor microenvironment studies |
| Picrosirius Red Staining | More sensitive for early collagen deposition | Early-stage fibrosis detection |
| Second-Harmonic Generation (SHG) Microscopy | Provides nonlinear optical imaging of collagen without staining | Advanced research applications |
🔍 8. Comparison with Other Staining Techniques
Understanding how Masson’s Trichrome stain compares with other histological stains is crucial for selecting the appropriate method in research and clinical diagnostics. Different staining techniques highlight various tissue components and are chosen based on the diagnostic or investigative need.
🧪 A. Masson’s Trichrome Stain: A Quick Recap
- Primary Purpose: Differentiates collagen fibers (blue or green), muscle fibers (red), and cell nuclei (black).
- Applications: Assessing fibrosis, tumor invasion, and tissue architecture in cancer and fibrotic diseases.
- Strengths: Excellent for visualizing connective tissue and muscle differentiation.
- Limitations: Cannot distinguish collagen subtypes and lacks cellular specificity.
⚖️ B. Key Comparisons with Other Staining Techniques
| Stain | Tissue Components Highlighted | Advantages | Limitations | Ideal For |
|---|---|---|---|---|
| Masson’s Trichrome | Collagen (blue/green), Muscle (red), Nuclei (black) | Clear contrast between muscle and collagen | No collagen subtype differentiation | Fibrosis, Tumor invasion |
| Hematoxylin & Eosin (H&E) | Nuclei (blue/purple), Cytoplasm (pink) | Routine diagnostic stain, shows general morphology | Poor differentiation of connective tissue | General pathology, Cancer staging |
| Sirius Red | Collagen I & III (red/yellow under polarized light) | Differentiates collagen subtypes, high sensitivity | Requires polarized microscopy | Liver fibrosis, Cardiovascular research |
| Periodic Acid–Schiff (PAS) | Glycogen, mucin, basement membranes (magenta) | Highlights carbohydrates and basement membranes | Cannot differentiate collagen or muscle | Kidney pathology, Tumor basement membranes |
| Van Gieson Stain | Collagen (red), Muscle (yellow), Nuclei (black) | Quick procedure, strong contrast for collagen | Less precise for muscle tissue compared to Masson’s | Collagen visualization, Cardiovascular tissues |
| Gomori’s Trichrome | Collagen (green), Muscle (red), Nuclei (black) | Good for muscle fiber assessment | Less widely used in cancer studies | Muscle pathology, Mitochondrial diseases |
| Reticulin Stain (Silver Stain) | Reticular fibers (black) | Highlights reticular framework in tissues | Limited to reticular fibers, more expensive | Lymphoma diagnosis, Liver architecture |
| Alcian Blue | Acidic mucopolysaccharides (blue) | Identifies mucins in tumors | Does not stain collagen or muscle | Mucinous carcinoma, Gastrointestinal pathology |
| Elastic Stains (Verhoeff-Van Gieson) | Elastic fibers (black), Collagen (red) | Visualizes elastic fibers in vessel walls | Less useful for tumor tissue analysis | Vascular pathology, Lung disease |
🔬 C. Masson’s Trichrome vs. Sirius Red in Fibrosis
| Feature | Masson’s Trichrome | Sirius Red |
|---|---|---|
| Sensitivity | Moderate for collagen | High sensitivity, especially for early fibrosis |
| Collagen Subtype Distinction | Does not differentiate subtypes | Differentiates collagen I (red) & III (green/yellow) under polarized light |
| Quantification | Qualitative assessment | Quantifiable with image analysis software |
| Application | General fibrosis detection | Detailed fibrosis staging (e.g., liver fibrosis scoring) |
🏥 D. Masson’s Trichrome vs. H&E in Cancer Diagnostics
| Aspect | Masson’s Trichrome | Hematoxylin & Eosin (H&E) |
|---|---|---|
| Tumor-Stroma Interaction | Clearly highlights stromal collagen | Limited visibility of stromal components |
| Cancer Invasion Analysis | Shows tumor invasion into collagen-rich stroma | Less effective in differentiating invasion boundaries |
| Routine Use | Specialized stain for connective tissue | Gold standard for initial cancer diagnosis |
| Resolution of Cellular Details | Moderate | High (better nuclear and cytoplasmic detail) |
🌟 E. Unique Advantages of Masson’s Trichrome
- Provides distinct separation between muscle tissue and collagen, aiding in the staging of invasive cancers such as bladder and breast carcinomas.
- Particularly useful for assessing fibrotic encapsulation around tumors, a factor influencing tumor progression and therapy resistance.
- Essential in myocardial fibrosis detection following infarction, where collagen replacement impacts cardiac function.
🚫 F. When to Choose Other Stains Over Masson’s Trichrome
- Need for Early Fibrosis Detection: Opt for Sirius Red for higher sensitivity.
- Collagen Subtype Analysis: Choose Sirius Red or immunohistochemistry (IHC) for specific collagen subtypes.
- Detailed Nuclear Morphology: Use H&E for precise cellular detail, crucial in initial tumor grading.
- Mucinous Tumors: Alcian Blue provides better mucin visualization.
- Vascular Changes: Elastic stains such as Verhoeff-Van Gieson are superior for vascular pathology.
📝 G. Integrating Multiple Stains for Comprehensive Diagnosis
In many cases, a multi-stain approach provides the most comprehensive insights:
- H&E + Masson’s Trichrome: For cancer tissue architecture and stromal invasion analysis.
- Sirius Red + Masson’s Trichrome: For in-depth fibrosis staging and collagen analysis.
- Masson’s Trichrome + Immunohistochemistry (IHC): For combining structural and molecular information, particularly in oncology research.
❓ 9. Frequently Asked Questions (FAQs)
Q1: How does Masson’s Trichrome stain help in fibrosis detection?
Masson’s Trichrome stain is particularly effective for detecting fibrosis due to its ability to highlight collagen fibers in blue or green, while other tissue components like muscle fibers and cytoplasm stain red. The stain allows for clear differentiation between normal tissue and areas where collagen has accumulated due to fibrotic changes. This makes it a valuable tool in assessing the extent of fibrosis in organs such as the liver, lungs, heart, and kidneys. The intensity and distribution of collagen can help pathologists stage fibrosis and monitor its progression in chronic conditions like cirrhosis, pulmonary fibrosis, and cardiac fibrosis.
Q2: What are the critical steps in the staining process?
The key steps in the Masson’s Trichrome staining protocol are as follows:
- Fixation and Tissue Preparation
- Tissue is fixed in neutral-buffered formalin and embedded in paraffin. Thin sections (4-5 µm) are then cut.
- Deparaffinization and Rehydration
- Paraffin is removed with xylene, and tissue sections are rehydrated through graded alcohols (100% to 70%).
- Staining with Weigert’s Hematoxylin
- Nuclei are stained with Weigert’s iron hematoxylin, which stains them a dark blue or black.
- Biebrich Scarlet-Acid Fuchsin Staining
- Muscle fibers and cytoplasm are stained red with Biebrich scarlet-acid fuchsin.
- Differentiation with Phosphomolybdic/Phosphotungstic Acid
- The sections are briefly incubated in phosphomolybdic or phosphotungstic acid, which removes excess red stain from non-collagenous tissues.
- Collagen Staining with Aniline Blue or Light Green
- Collagen fibers are stained blue or green, providing the necessary contrast for fibrosis detection.
- Dehydration, Clearing, and Mounting
- The slides are dehydrated through graded alcohols and cleared in xylene before being mounted with a coverslip.
Each of these steps plays a crucial role in ensuring that the tissue is properly stained and that the final results are clear and interpretable.
Q3: How do you interpret staining results in different tissues?
Interpreting Masson’s Trichrome staining results requires careful observation of the colors that correspond to different tissue components:
- Collagen:
- Stains blue or green (depending on the dye used). The presence of blue/green areas indicates collagen deposition, which is particularly relevant in detecting fibrosis or fibrotic scars.
- In cancerous tissues, collagen can be seen in the tumor stroma, marking tumor invasion.
- Muscle:
- Stains red. The intensity of red staining helps assess muscle tissue and its involvement in processes like muscle atrophy or tumor-muscle interactions.
- Nuclei:
- Stains black or dark blue with Weigert’s hematoxylin. The sharp delineation of nuclei allows for the analysis of cellular architecture and tissue organization.
Interpretation by Tissue Type:
- Liver:
- In liver fibrosis, blue staining (collagen) around the hepatocytes indicates the degree of fibrotic change. The pattern of fibrosis can suggest the stage of liver disease (e.g., cirrhosis).
- Heart:
- Fibrosis in the heart (e.g., after myocardial infarction) will present as blue/green regions in the heart wall, particularly in the myocardial interstitial areas.
- Lungs:
- In lung fibrosis, the blue staining of collagen in the interstitial tissue marks the extent of fibrotic remodeling, which can help assess the severity of diseases like pulmonary fibrosis.
- Kidney:
- In renal tissue, blue collagen staining highlights glomerulosclerosis and tubulointerstitial fibrosis, which are hallmarks of chronic kidney disease.
In each tissue, the intensity and distribution of collagen staining provide valuable information about the extent of fibrosis or tissue remodeling, aiding in disease diagnosis and staging.
Conclusion
Masson’s Trichrome stain is a powerful histological tool for differentiating between collagen, muscle fibers, and cell nuclei, making it invaluable for assessing tissue fibrosis and tumor progression. Its ability to highlight the structural changes in connective tissue provides essential insights into various diseases, including cancer, fibrosis, and cardiovascular disorders. By understanding the staining protocol, applications, and interpretations, researchers and pathologists can use Masson’s Trichrome stain to enhance their diagnostic and research capabilities, ensuring accurate disease staging and monitoring.