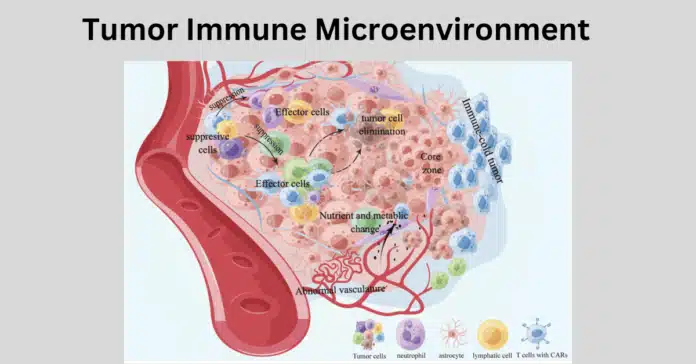
The tumor immune microenvironment plays a pivotal role in cancer progression, influencing how tumors grow, invade, and evade the immune system. Within this complex ecosystem, immune cells interact with cancer cells, either suppressing tumor growth or inadvertently aiding its progression.
Understanding the tumor immune microenvironment is critical for uncovering new therapeutic strategies, as it holds the key to unlocking the body’s natural defenses against cancer.
In this blog post, we’ll explore the key components, mechanisms of immune evasion, and innovative therapies targeting this dynamic microenvironment.
What is the Tumor Immune Microenvironment?
Definition and Importance
The tumor immune microenvironment (TIME) refers to the dynamic and complex ecosystem surrounding a tumor, which consists of various cell types, signaling molecules, and extracellular components. It plays a crucial role in shaping tumor behavior, influencing both its progression and response to therapy.
The TIME is primarily composed of:
- Immune Components: Immune cells such as T-cells, tumor-associated macrophages (TAMs), dendritic cells (DCs), natural killer (NK) cells, and myeloid-derived suppressor cells (MDSCs).
- Non-Immune Components: Cancer-associated fibroblasts (CAFs), stromal cells, blood vessels, and the extracellular matrix (ECM).
The interaction between these components in the tumor microenvironment creates a highly adaptable environment, allowing tumors to evade immune detection while facilitating growth and metastasis.
Role in Cancer Development
The tumor immune microenvironment is a double-edged sword in cancer development. On one hand, it supports immune surveillance, where the body’s immune cells detect and destroy abnormal cells. On the other hand, tumors can manipulate the TIME to suppress immune responses, promoting survival and growth.
- Immune Evasion Mechanisms
- Checkpoint Pathway Activation: Tumors exploit immune checkpoints such as PD-1/PD-L1 and CTLA-4 to inhibit T-cell activity.
- Cytokine Secretion: Tumors secrete immunosuppressive cytokines like IL-10 and TGF-β to dampen anti-tumor immunity.
- Recruitment of Suppressive Cells: MDSCs and regulatory T cells (Tregs) are attracted to the TIME, where they suppress immune activity.
- Immune Surveillance and Tumor Immunoediting
Tumor immunoediting describes the process by which the immune system shapes tumor development through three phases:- Elimination: Immune cells recognize and destroy tumor cells.
- Equilibrium: Some tumor cells survive and enter a dormant state.
- Escape: Tumor cells adapt and evade immune detection, leading to progression.
Key Players in the Tumor Immune Microenvironment
The TIME is populated by diverse immune and stromal cells, each playing distinct roles:
- Immune Cells:
- T-Cells: Tumor-infiltrating lymphocytes (TILs) can either attack tumor cells (cytotoxic T-cells) or suppress immunity (Tregs).
- Tumor-Associated Macrophages (TAMs): TAMs exhibit dual roles, with M1 macrophages acting as anti-tumor agents and M2 macrophages promoting tumor growth and immune suppression.
- Natural Killer (NK) Cells: NK cells attack tumor cells directly but may become dysfunctional within the TIME.
- Stromal Cells:
- Cancer-Associated Fibroblasts (CAFs): CAFs remodel the extracellular matrix and secrete factors that support tumor growth and immune evasion.
- Endothelial Cells: These form new blood vessels, facilitating tumor angiogenesis and providing nutrients.
- Signaling Molecules:
- Cytokines and Chemokines: These molecules regulate immune cell recruitment and activity, often tipping the balance in favor of tumor survival.
Immune Cells and Their Roles in the Tumor Immune Microenvironment
The tumor immune microenvironment (TIME) is a dynamic battlefield where various immune cells either combat or support tumor growth. These cells play complex and sometimes contradictory roles, shaping the tumor’s progression and response to therapy.
Tumor-Associated Macrophages (TAMs)
Macrophages are among the most abundant immune cells in the TIME, where they exhibit dual roles depending on their polarization state:
- Pro-Tumor vs. Anti-Tumor Roles
- M1 Macrophages (Anti-Tumor): These are activated by pro-inflammatory signals and can directly kill tumor cells by releasing cytotoxic substances such as nitric oxide.
- M2 Macrophages (Pro-Tumor): These are often co-opted by tumors to suppress immune responses, promote tissue remodeling, and support angiogenesis.
- Impact on Tumor Progression and Metastasis
- M2-like TAMs secrete cytokines and growth factors, such as VEGF and TGF-β, that stimulate tumor growth, enhance blood vessel formation, and facilitate metastasis.
- TAMs also contribute to immune evasion by inhibiting the activity of cytotoxic T-cells and natural killer (NK) cells within the tumor microenvironment.
T-Cells in the Tumor Immune Microenvironment
T-cells are central to anti-tumor immunity, but their effectiveness is often compromised within the TIME.
- Tumor-Infiltrating Lymphocytes (TILs)
- Cytotoxic T-Cells (CD8+ T-cells): These cells directly kill tumor cells by recognizing cancer-specific antigens. High levels of TILs are often associated with better clinical outcomes.
- Regulatory T-Cells (Tregs): Tregs suppress immune responses, preventing effective anti-tumor activity and supporting tumor immune evasion.
- T-Cell Exhaustion and Immune Checkpoints
- Persistent antigen exposure in the TIME leads to T-cell exhaustion, characterized by reduced functionality and proliferation.
- Immune checkpoint molecules such as PD-1 and CTLA-4 further inhibit T-cell activity, allowing tumors to escape immune destruction.
Myeloid-Derived Suppressor Cells (MDSCs)
MDSCs are a heterogeneous population of immature myeloid cells that accumulate in the TIME, where they play key immunosuppressive roles.
- Suppression of Immune Response
- MDSCs inhibit the activation and function of T-cells and NK cells by releasing suppressive molecules such as arginase-1 and reactive oxygen species (ROS).
- Contribution to Immune Evasion
- By suppressing anti-tumor immunity, MDSCs enable tumors to grow unchecked and resist immune-based therapies.
- They also promote angiogenesis and facilitate metastasis, further aiding tumor progression.
Natural Killer (NK) Cells
NK cells are innate immune cells capable of killing tumor cells without prior sensitization. However, their activity can be inhibited within the TIME.
- Anti-Tumor Activity
- NK cells recognize and destroy tumor cells by detecting stress-induced ligands on their surface, releasing cytotoxic granules, and inducing apoptosis.
- They also produce cytokines like IFN-γ, which enhance the anti-tumor activity of other immune cells.
- Regulation in the Tumor Microenvironment
- Tumors release soluble factors such as TGF-β that suppress NK cell activity.
- The hypoxic conditions of the TIME further impair NK cell cytotoxicity, reducing their effectiveness.
Each immune cell type in the TIME plays a nuanced role, with the balance between pro-tumor and anti-tumor activities determining the overall outcome. Understanding these interactions is crucial for developing therapies that reprogram the TIME to favor anti-tumor immunity.
Mechanisms of Immune Evasion in Tumors
Cancer cells employ diverse mechanisms to evade immune detection and suppression, ensuring their survival and progression. These strategies involve manipulating immune checkpoint pathways, secreting immunosuppressive factors, and creating a hypoxic environment that further inhibits immune responses.
Checkpoint Pathways
Immune checkpoint pathways are regulatory mechanisms designed to prevent excessive immune responses. However, tumors exploit these pathways to suppress anti-tumor immunity:
- Role of PD-1/PD-L1 and CTLA-4 in Immune Suppression
- PD-1/PD-L1 Axis: Tumor cells and surrounding stromal cells often express PD-L1, which binds to PD-1 receptors on T-cells. This interaction prevents T-cell activation, allowing tumor cells to evade destruction.
- CTLA-4 Pathway: CTLA-4, expressed on T-cells, competes with CD28 for binding to B7 ligands on antigen-presenting cells (APCs). Tumors exploit this pathway to dampen T-cell activation early in the immune response.
- Strategies Tumors Use to Exploit These Pathways
- Upregulation of PD-L1 expression in response to inflammatory cytokines like IFN-γ, creating an immunosuppressive shield.
- Recruiting immune cells like regulatory T-cells (Tregs) to amplify checkpoint-mediated suppression.
Tumor-Derived Factors
Tumor cells and associated stromal cells produce a range of factors that modulate immune activity and create a suppressive microenvironment:
- Cytokines and Chemokines
- Cytokines: IL-6 and IL-10 are often secreted by tumor cells to suppress dendritic cell maturation, inhibit T-cell proliferation, and promote the differentiation of suppressive immune cells like myeloid-derived suppressor cells (MDSCs).
- Chemokines: CXCL12 recruits immunosuppressive cells such as Tregs and MDSCs into the tumor microenvironment, further dampening anti-tumor immune responses.
- Impact on Immune Cell Recruitment and Suppression
- Tumor-derived cytokines not only suppress effector immune cells but also polarize macrophages into a pro-tumor (M2) phenotype.
- Chemokines like CCL2 facilitate the recruitment of monocytes that differentiate into tumor-supportive macrophages.
Hypoxia and Immune Suppression
The tumor microenvironment is frequently hypoxic due to abnormal vasculature and rapid tumor growth. Hypoxia contributes to immune evasion in several ways:
- Role of Hypoxia-Inducible Factors (HIFs)
- HIF-1α and HIF-2α are transcription factors activated under low oxygen conditions. These factors drive the expression of VEGF to promote angiogenesis and CXCL12 to recruit suppressive immune cells.
- HIFs also induce PD-L1 expression on tumor cells, enhancing immune checkpoint-mediated suppression.
- Interaction with the Extracellular Matrix (ECM) and Angiogenesis
- Hypoxia alters the ECM composition, increasing the deposition of collagen and other structural proteins. This remodeling creates physical barriers that impede immune cell infiltration into tumors.
- The hypoxic environment promotes the formation of abnormal blood vessels, which are inefficient in delivering oxygen and immune cells, further reducing the effectiveness of anti-tumor responses.
Targeting the Tumor Immune Microenvironment in Cancer Therapy
Advances in cancer research have shifted focus toward the tumor immune microenvironment (TIME) as a therapeutic target. By reprogramming immune cells or disrupting tumor-induced immune suppression, these approaches aim to restore anti-tumor immunity and improve patient outcomes.
Immune Checkpoint Inhibitors
Checkpoint inhibitors have revolutionized cancer therapy, especially in cancers like melanoma, lung cancer, and bladder cancer:
- Success Stories of PD-1 and CTLA-4 Inhibitors
- Drugs like pembrolizumab (anti-PD-1) and ipilimumab (anti-CTLA-4) have shown remarkable efficacy in treating advanced cancers, with some patients experiencing durable responses.
- Their ability to “unleash” T-cells has been instrumental in improving survival rates for cancers previously deemed intractable.
- Challenges and Resistance Mechanisms
- Many patients fail to respond due to inherent or acquired resistance. Mechanisms include upregulation of alternate checkpoints (e.g., TIM-3, LAG-3), immunosuppressive metabolites, or lack of immunogenic tumor antigens.
- The dense extracellular matrix in certain tumors can physically hinder drug delivery, reducing effectiveness.
CAR-T Cell Therapy
Chimeric antigen receptor (CAR)-T cell therapy has emerged as a transformative approach, particularly for hematological malignancies:
- Role of Engineered T-Cells in Targeting Tumors
- CAR-T cells are genetically modified to recognize specific tumor antigens, enabling precise targeting of cancer cells. This therapy has shown significant success in treating B-cell malignancies by targeting CD19.
- Innovations in CAR design, such as introducing co-stimulatory domains, enhance T-cell survival and function in the TIME.
- Limitations and Future Directions
- CAR-T therapies face challenges in solid tumors, including poor infiltration into the TIME, antigen heterogeneity, and immunosuppressive signals.
- Future advancements may include “armored CAR-T cells” that secrete cytokines to counteract immunosuppression or dual-targeting CARs to address antigen variability.
Combination Therapies
Combining therapies has proven effective in overcoming the limitations of monotherapies and enhancing treatment efficacy:
- Synergistic Approaches
- Pairing immune checkpoint inhibitors with chemotherapy or radiotherapy can increase tumor antigen release, improving immune activation.
- Combining checkpoint inhibitors with anti-angiogenic therapies can normalize the tumor vasculature, improving immune cell infiltration.
- Enhancing Efficacy While Minimizing Side Effects
- The combination of immune-based therapies with low-dose chemotherapy reduces toxicity while maintaining efficacy.
- Rational design of combination regimens is key to avoiding overlapping toxicities and immune-related adverse events.
Emerging Therapies for Tumor Immune Microenvironment
Innovative strategies targeting the TIME are on the horizon, offering new possibilities for cancer treatment:
- Therapeutic Vaccines
- Vaccines targeting TIME-associated antigens, such as those expressed by stromal cells or TAMs, aim to enhance immune recognition of the tumor.
- Personalized cancer vaccines based on neoantigens identified in individual patients are also under investigation.
- Role of Exosomes and Extracellular Vesicles
- Exosomes secreted by tumor cells carry immunosuppressive signals, but engineered exosomes are being explored as delivery vehicles for immunostimulatory molecules.
- Extracellular vesicles loaded with RNA or proteins can modulate the TIME, reactivating anti-tumor immune responses.
Targeting the tumor immune microenvironment has unlocked a new era in cancer immunotherapy. By addressing both the cellular and molecular dynamics of the TIME, these therapies hold the promise of more effective and personalized cancer treatments.
Future Perspectives and Challenges
The tumor immune microenvironment (TIME) presents both opportunities and challenges in advancing cancer therapy. Future research and innovation aim to overcome current limitations by personalizing treatments, identifying reliable biomarkers, tackling resistance mechanisms, and employing advanced models for better therapeutic insights.
Personalized Medicine
The heterogeneity of the TIME demands a move toward highly individualized cancer therapies:
- Tailoring Therapies to Specific Tumor Immune Profiles
- Advances in genomic, transcriptomic, and proteomic profiling enable detailed characterization of a tumor’s immune landscape. This allows therapies to be customized for a patient’s specific immune context, improving efficacy.
- Techniques like single-cell RNA sequencing reveal interactions between tumor and immune cells, guiding the choice of immune checkpoint inhibitors, CAR-T therapies, or combination approaches.
Biomarkers in the Tumor Microenvironment
Predictive and prognostic biomarkers are essential for selecting the right therapies and monitoring their success:
- Identifying Predictive Markers for Therapy Response
- PD-L1 expression levels and tumor mutational burden (TMB) have shown promise as indicators of responsiveness to immune checkpoint inhibitors.
- Emerging biomarkers, such as immune gene signatures, cytokine profiles, or specific immune cell populations (e.g., CD8+ T-cells), may further refine patient selection.
- Liquid biopsies, which detect circulating tumor DNA (ctDNA) and immune-derived exosomes, offer a non-invasive approach to monitor changes in the TIME during treatment.
Overcoming Resistance
Resistance to immune-based therapies remains a significant challenge in clinical oncology:
- Addressing Adaptive Resistance in Immune-Targeting Therapies
- Tumors can develop adaptive resistance by upregulating alternative immune checkpoints (e.g., TIM-3, LAG-3) or altering antigen presentation. Developing combination therapies targeting multiple pathways simultaneously is a promising approach.
- Epigenetic modifications within the TIME can suppress immune activation. Drugs targeting these epigenetic changes (e.g., HDAC inhibitors) are being explored to restore immune responsiveness.
- Strategies to enhance immune cell infiltration into the tumor, such as targeting the extracellular matrix or normalizing tumor vasculature, are critical to overcoming physical barriers.
Role of Advanced 3D Cell Culture Models
Traditional 2D cell cultures fail to replicate the complexity of the TIME. Advanced 3D models provide a more accurate platform for studying tumor-immune interactions:
- Simulating the Tumor Immune Microenvironment for Better Drug Testing
- Organoids and 3D bioprinting enable the creation of tumor models that include immune cells, stromal components, and vascular structures, closely mimicking in vivo conditions.
- Co-culture systems integrating tumor cells with immune cells allow researchers to study immune cell infiltration, checkpoint dynamics, and drug responses in a controlled setting.
- These models also support the testing of personalized therapies by incorporating patient-derived tumor and immune cells, bridging the gap between preclinical studies and clinical trials.
Conclusion
The tumor immune microenvironment plays a pivotal role in cancer progression and therapy response. Understanding its complex interactions offers valuable insights into immune evasion and provides opportunities for innovative therapeutic approaches. By leveraging strategies like immune checkpoint inhibitors, CAR-T cell therapy, and advanced combination therapies, alongside emerging technologies such as 3D models and personalized medicine, we can better harness the immune system to fight cancer. Continued research into this dynamic microenvironment holds the promise of transforming cancer care and improving patient outcomes.