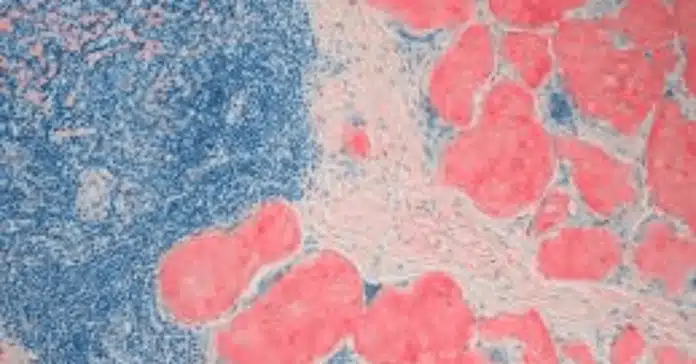
The Congo Red stain is a vital histological tool widely used in pathology and biomedical research, primarily for detecting amyloid deposits in tissue samples. Known for its unique ability to exhibit apple-green birefringence under polarized light, Congo Red stain plays a crucial role in diagnosing diseases such as amyloidosis and Alzheimer’s disease. Beyond amyloid detection, it is also applied in fungal identification and studying protein aggregation, making it indispensable in both clinical and research settings.
In this blog post, we will explore everything you need to know about Congo Red stain, including its chemical properties, applications in histology, and a step-by-step staining procedure. We will also discuss how to interpret staining results, compare it with other amyloid staining techniques, and examine the advantages, limitations, and latest research involving Congo Red staining.
What is Congo Red Stain?
Congo Red stain is a synthetic dye primarily used in histology and pathology for the detection of amyloid deposits in tissues. It belongs to the azo dye family, characterized by the presence of azo groups (-N=N-) that link aromatic compounds, giving the dye its distinctive red color. First introduced in the late 19th century, Congo Red gained prominence due to its unique ability to bind selectively to amyloid fibrils, making it an essential diagnostic tool in various medical and research applications.
🔬 Chemical Structure and Properties
Congo Red is a benzidine-based diazo dye with a linear, planar structure that allows it to intercalate between β-pleated sheets in amyloid fibrils. This interaction is the basis for its diagnostic use, as the dye aligns parallel to these sheets, resulting in a characteristic apple-green birefringence when viewed under polarized light.
Key properties of Congo Red dye include:
- Color: Red in solution, with green birefringence under polarized light when bound to amyloid.
- Solubility: Water-soluble, which facilitates its use in histological staining.
- Staining Behavior: Exhibits high specificity for amyloid fibrils due to hydrogen bonding and hydrophobic interactions with β-pleated sheet structures.
🧪 Mechanism of Action: How Congo Red Binds to Amyloid
Congo Red’s diagnostic power lies in its ability to bind specifically to amyloid proteins, which are characterized by β-pleated sheet secondary structures. The dye molecules attach to these sheets through a combination of:
- Hydrogen bonding between the dye’s azo groups and the peptide backbone of the amyloid fibrils.
- Hydrophobic interactions that stabilize the dye-fibril complex.
This binding not only stains the amyloid deposits red but also causes them to exhibit apple-green birefringence under polarized light, a hallmark feature used for diagnostic purposes.
🏥 Why Congo Red Stain is Important
Congo Red stain plays a critical role in the diagnosis of:
- Amyloidosis: A condition characterized by abnormal amyloid deposits in organs and tissues.
- Alzheimer’s Disease: Detection of amyloid plaques in brain tissues.
- Fungal Infections: Identification of fungal cell walls in microbiological studies.
Its specificity and diagnostic reliability make it indispensable in both clinical pathology and biomedical research.
In the next sections, we will explore the various applications of Congo Red stain in greater detail, outline the complete staining procedure, and discuss how to interpret the results effectively.
Applications of Congo Red Stain
The Congo Red stain is widely used in histology, pathology, and microbiology due to its ability to bind selectively to specific biological structures. Its unique staining properties, especially the characteristic apple-green birefringence under polarized light when bound to amyloid fibrils, make it an essential diagnostic and research tool. Below are the key applications of Congo Red stain:
🏥 Amyloid Detection in Histology
The most notable application of Congo Red stain is in the detection of amyloid deposits in tissue samples. Amyloidosis, a condition where amyloid proteins accumulate abnormally in organs and tissues, can lead to severe organ dysfunction. Congo Red staining enables pathologists to:
- Visualize amyloid deposits: Amyloid appears as red deposits under light microscopy and exhibits apple-green birefringence when viewed under polarized light, a hallmark of amyloid presence.
- Diagnose amyloidosis and Alzheimer’s disease: Amyloid plaques in the brain are a key feature of Alzheimer’s disease, and Congo Red staining plays a crucial role in confirming such diagnoses.
- Differentiate amyloid from other tissue components: The specificity of Congo Red for β-pleated sheet structures makes it reliable for distinguishing amyloid from other protein aggregates.
🍄 Fungal Cell Wall Identification
Congo Red stain is also used in mycology for identifying fungal elements:
- Staining fungal cell walls: It binds to polysaccharides in fungal cell walls, helping to visualize fungi in tissue sections and culture preparations.
- Studying fungal morphogenesis: Researchers use Congo Red to observe changes in fungal cell walls during growth and development, providing insights into fungal biology and potential antifungal targets.
🔬 Protein Aggregation Studies in Research
Congo Red’s ability to bind to β-pleated sheet structures makes it valuable in studying protein aggregation, which is central to many diseases, including neurodegenerative disorders like:
- Alzheimer’s disease (amyloid-β plaques)
- Parkinson’s disease (α-synuclein aggregates)
- Huntington’s disease (huntingtin protein aggregation)
In these studies, Congo Red helps researchers:
- Track the formation and growth of protein aggregates.
- Test potential therapeutic agents that prevent or reverse aggregation.
🏛️ Biomedical and Industrial Uses
Beyond medical and research applications, Congo Red is used in:
- Cellulose and polysaccharide research: Due to its affinity for polysaccharides, Congo Red is applied in studies of plant cell walls and biofilms.
- Material science: Used to detect amyloid-like fibrils in synthetic materials, contributing to biomaterial development.
- Pharmaceutical research: Screening for compounds that can disrupt amyloid formation, potentially leading to treatments for amyloid-related diseases.
💉 Diagnostic Applications in Pathology
Congo Red stain is crucial for diagnosing:
- Primary and secondary amyloidosis
- Hereditary amyloidosis
- Localized amyloidosis associated with tumors
- Amyloid-related systemic diseases affecting the heart, kidneys, liver, and nervous system
Its ability to provide rapid, reliable results makes it a first-line staining method in pathology labs.
📝 Congo Red in Polarized Light Microscopy
One of the most distinguishing features of Congo Red stain is the apple-green birefringence observed when stained amyloid is viewed under polarized light microscopy. This optical property is critical because:
- It confirms the presence of amyloid fibrils with high specificity.
- It helps differentiate amyloid deposits from other substances that might also appear red under regular light microscopy.
🌐 Congo Red in Advanced Imaging Techniques
Recent advancements have expanded the use of Congo Red in:
- Fluorescence microscopy, where Congo Red can emit fluorescence when bound to amyloid fibrils, enhancing detection sensitivity.
- Electron microscopy, providing ultrastructural details of amyloid deposits when combined with other staining techniques.
In the next section, we will provide a detailed, step-by-step Congo Red staining procedure, highlighting the materials required, best practices, and troubleshooting tips to ensure accurate and reliable results.
Congo Red Staining Procedure
The Congo Red staining procedure is essential for the accurate detection of amyloid deposits in tissue sections. Proper execution of this method allows pathologists and researchers to observe the characteristic apple-green birefringence under polarized light, a hallmark of amyloid presence. Below is a step-by-step guide outlining the materials required, staining protocol, and tips for optimal results.
🧪 Materials and Reagents Required
To perform Congo Red staining, you will need the following:
- Tissue sections: Formalin-fixed, paraffin-embedded tissue sections (5–10 µm thick)
- Congo Red staining solution: Typically prepared as 1% Congo Red in an alkaline alcoholic solution
- Alkaline salt solution: Often saturated sodium chloride in 80% ethanol, adjusted to pH 10
- Hematoxylin solution: For nuclear counterstaining
- Ethanol series: 80%, 95%, and absolute ethanol for dehydration
- Xylene: For clearing the tissue sections
- Mounting medium and coverslips
- Polarized light microscope
📝 Step-by-Step Staining Protocol
🔹 Step 1: Deparaffinization and Rehydration
- Place the tissue slides in xylene for 5–10 minutes to remove paraffin.
- Rehydrate the slides by passing them through a graded series of ethanol (100%, 95%, and 80%) for 2 minutes each.
- Rinse the slides thoroughly in distilled water.
🔹 Step 2: Staining with Congo Red
- Immerse the slides in the alkaline salt solution for 20 minutes. This step enhances the specificity of Congo Red binding to amyloid fibrils.
- Stain the tissue sections in Congo Red solution for 30–60 minutes. The duration may vary depending on tissue type and amyloid concentration.
- Rinse the slides quickly in distilled water to remove excess stain.
🔹 Step 3: Counterstaining
- Counterstain with hematoxylin for 1–2 minutes to highlight nuclei, providing better contrast for amyloid deposits.
- Rinse briefly in tap water and then in distilled water.
🔹 Step 4: Dehydration and Clearing
- Dehydrate the stained sections by passing them through 95% and absolute ethanol (2 minutes each).
- Clear the sections in xylene for 5 minutes.
🔹 Step 5: Mounting
- Apply a few drops of mounting medium to the tissue section.
- Gently place a coverslip over the tissue, avoiding air bubbles.
- Allow the slides to dry completely before microscopic examination.
🔬 Microscopic Examination
- Examine the slides under a polarized light microscope.
- Positive amyloid deposits will appear red under normal light and display apple-green birefringence under polarized light—this birefringence is diagnostic of amyloid.
- If birefringence is not observed, check the staining protocol for potential errors, such as inadequate staining time or incorrect pH levels.
⚡ Troubleshooting Tips
- No birefringence observed? Ensure the pH of the alkaline salt solution is correctly adjusted (pH 10 is optimal).
- Weak staining? Extend the staining time with Congo Red solution or confirm the tissue fixation quality.
- Non-specific staining? Prolong the washing steps or verify the purity of Congo Red dye used.
- Fading of birefringence: Always use freshly prepared reagents and minimize exposure to bright light before examination.
💡 Safety Considerations
- Congo Red is potentially hazardous; handle with gloves and work in a well-ventilated area.
- Dispose of chemical waste properly, following institutional safety guidelines.
- Always wear appropriate personal protective equipment (PPE), including lab coats and safety goggles.
🎯 Summary of the Staining Process
| Step | Duration | Purpose |
|---|---|---|
| Deparaffinization | 5–10 minutes | Remove paraffin from tissues |
| Rehydration | 2 minutes/step | Prepare tissue for staining |
| Congo Red staining | 30–60 minutes | Stain amyloid deposits |
| Hematoxylin counterstain | 1–2 minutes | Highlight nuclei for contrast |
| Dehydration & Clearing | 2–5 minutes | Prepare for permanent mounting |
| Mounting & Drying | Until dry | Finalize slides for microscopy |
In the next section, we will explore how to interpret Congo Red staining results, including key diagnostic features, potential pitfalls, and how to differentiate true amyloid deposits from artifacts.
Interpretation of Congo Red Stain Results
The interpretation of Congo Red stain results is a crucial step in diagnosing amyloid-related diseases and understanding protein aggregation in research settings. The hallmark of a positive Congo Red stain is the appearance of apple-green birefringence when viewed under polarized light. Accurate interpretation ensures correct diagnosis and guides further clinical or research decisions.
🔍 Visual Characteristics Under Microscopy
🧭 Under Brightfield (Light) Microscopy
- Positive Result: Amyloid deposits appear as orange-red to salmon-pink areas in the tissue. These deposits are typically extracellular and may be found around blood vessels, in connective tissue, or within organ parenchyma.
- Negative Result: No red staining in expected amyloid locations. Tissue components will only show the nuclear counterstain (blue/purple from hematoxylin).
🌈 Under Polarized Light Microscopy
- Positive Result: The diagnostic feature is the apple-green birefringence of amyloid deposits. This green coloration results from the ordered alignment of Congo Red molecules with the β-pleated sheet structure of amyloid fibrils.
- Negative Result: Absence of green birefringence suggests no amyloid presence or a technical issue with staining.
🎨 Key Features of Positive Congo Red Staining
- Apple-Green Birefringence: Specific for amyloid fibrils due to their β-pleated sheet structure.
- Extracellular Localization: Amyloid deposits are usually found outside cells, often surrounding blood vessels or replacing organ parenchyma.
- Consistency Across Sections: Multiple sections should consistently show birefringence in the same regions for a confident diagnosis.
⚠️ Differentiating True Positives from Artifacts
🚫 Common Artifacts and How to Identify Them:
- Non-Specific Staining: Some non-amyloid structures, such as collagen or elastin, may appear red under brightfield but do not exhibit apple-green birefringence under polarized light.
- Tissue Folding: Overlapping tissue folds may mimic amyloid deposition. Ensure proper sectioning and slide preparation.
- Autofluorescence: In fluorescence microscopy, some tissues may naturally fluoresce; confirm amyloid presence via polarized light birefringence.
💊 Clinical Implications of Positive Congo Red Staining
🏥 Amyloidosis Diagnosis
A positive Congo Red stain is often a key diagnostic criterion for various forms of amyloidosis, including:
- AL (Primary) Amyloidosis: Associated with plasma cell dyscrasias; amyloid derived from immunoglobulin light chains.
- AA (Secondary) Amyloidosis: Results from chronic inflammatory conditions; amyloid derived from serum amyloid A protein.
- ATTR (Transthyretin) Amyloidosis: Linked to hereditary conditions or aging; amyloid derived from transthyretin protein.
- Localized Amyloidosis: Occurs in specific organs, such as the brain in Alzheimer’s disease (amyloid-β plaques).
🧬 Alzheimer’s Disease
- Congo Red staining highlights amyloid-β plaques in the brain, a hallmark of Alzheimer’s pathology.
- The intensity and distribution of amyloid deposits correlate with disease progression.
🔬 Quantitative Analysis of Congo Red Staining
For research purposes, quantitative methods can assess amyloid burden:
- Image Analysis Software: Used to measure the area and intensity of Congo Red staining.
- Spectrophotometric Methods: Assess Congo Red binding in solution to quantify amyloid concentration.
- Birefringence Intensity Scoring: Pathologists may score the degree of birefringence to estimate amyloid load.
🛠️ Troubleshooting and Considerations in Interpretation
- Weak Birefringence: May indicate insufficient staining time or incorrect pH in the staining solution.
- Loss of Birefringence: Overstaining or prolonged exposure to dehydration agents may fade the green birefringence.
- Tissue Processing Issues: Improper fixation can reduce staining quality; formalin fixation is recommended.
📋 Complementary Diagnostic Techniques
To confirm Congo Red staining results, additional methods may be employed:
- Immunohistochemistry (IHC): Detects specific amyloid proteins using antibodies.
- Mass Spectrometry: Precisely identifies amyloid protein subtypes.
- Thioflavin T/S Staining: Fluorescent dyes that bind to amyloid fibrils, offering complementary visualization.
- Electron Microscopy: Provides ultrastructural details of amyloid fibrils.
In the next section, we will delve into the advantages and limitations of Congo Red staining, comparing it with alternative amyloid detection methods and discussing how to overcome its challenges for optimal diagnostic outcomes.
Advantages and Limitations of Congo Red Stain
The Congo Red stain is considered the gold standard for detecting amyloid deposits due to its unique ability to produce apple-green birefringence under polarized light. While it remains a cornerstone in pathology and research, this staining method has both strengths and weaknesses that users must understand for accurate application and interpretation.
✅ Advantages of Congo Red Stain
🔬 Cost-Effective and Accessible
- Requires inexpensive reagents and standard laboratory equipment, including a polarized light microscope.
- Does not rely on expensive antibodies or specialized techniques, making it widely accessible for diagnostic labs.
⏱️ Rapid and Efficient
- The entire staining process can be completed in a few hours, enabling quick diagnostic turnaround for clinical cases.
- Suitable for routine screening in pathology labs.
💡 Minimal Equipment Requirements
- Only a polarized light microscope is necessary for confirming amyloid presence, making it suitable for resource-limited settings.
🔍 Visual Clarity
- Produces clear contrast between amyloid deposits and surrounding tissue, especially when paired with hematoxylin counterstain for nuclear visualization.
⚠️ Limitations of Congo Red Stain
🚫 1. Subjectivity in Interpretation
- The identification of apple-green birefringence can be observer-dependent, requiring experience to differentiate true positives from artifacts.
- Variations in microscope calibration and observer expertise may affect interpretation accuracy.
🧪 2. Sensitivity Limitations
- Small or sparse amyloid deposits may not be detected, particularly in early-stage disease.
- Congo Red staining may fail to identify amyloid in certain tissues with minimal deposition.
🎨 3. Potential for Artifacts
- Non-specific staining of collagen, elastin, or other tissue components may occur, leading to false positives.
- Tissue folding and processing issues can mimic amyloid deposition.
🧬 4. Lack of Amyloid Subtype Differentiation
- Congo Red staining cannot differentiate between amyloid types (e.g., AL vs. AA amyloidosis).
- Additional techniques, such as immunohistochemistry (IHC) or mass spectrometry, are required for subtype identification.
🕒 5. Birefringence Fading Over Time
- The apple-green birefringence can fade with prolonged storage, making retrospective analysis difficult.
- Freshly stained slides should be examined promptly for optimal results.
🧫 6. Technical Variability
- Results are highly sensitive to staining conditions, including:
- pH of staining solutions (optimal pH ~10)
- Staining duration
- Quality of tissue fixation (formalin-fixed tissues preferred)
- Small deviations can lead to weak staining or loss of birefringence.
🔍 7. Limited Quantitative Assessment
- Congo Red staining provides qualitative rather than quantitative information about amyloid burden.
- Quantitative methods, such as image analysis or spectrophotometry, are needed for precise measurement.
📝 Comparing Congo Red Stain with Alternative Techniques
| Technique | Advantages | Limitations |
|---|---|---|
| Congo Red Stain | High specificity; cost-effective; rapid | Observer-dependent; cannot subtype amyloid |
| Thioflavin T/S Staining | Fluorescent detection; higher sensitivity | Requires fluorescence microscopy; less specific |
| Immunohistochemistry (IHC) | Identifies amyloid subtypes; highly specific | Expensive; antibody-dependent; longer process |
| Mass Spectrometry | Gold standard for amyloid subtyping | High cost; requires specialized equipment |
| Electron Microscopy | Detailed ultrastructural analysis | Resource-intensive; not suitable for routine use |
🛠️ Overcoming Limitations: Best Practices
- Proper pH Control: Ensure the alkaline solution used for staining is at pH 10 for optimal dye-tissue interaction.
- Expert Training: Provide training for pathologists and lab technicians in polarized light microscopy interpretation.
- Use of Complementary Methods: For amyloid subtyping, combine Congo Red staining with IHC or mass spectrometry.
- Prompt Analysis: Examine stained slides promptly to avoid birefringence fading.
- Optimized Tissue Preparation: Ensure formalin fixation and proper sectioning (5–10 µm thickness) for consistent staining results.
🌟 Summary of Key Advantages and Limitations
| Advantages | Limitations |
|---|---|
| High specificity with birefringence | Observer-dependent interpretation |
| Cost-effective and accessible | Sensitivity issues with minimal deposits |
| Rapid and efficient procedure | Cannot differentiate amyloid subtypes |
| Minimal equipment needed | Technical variability affects outcomes |
| Clear visual contrast | Birefringence may fade over time |
In the next section, we will explore the comparison of Congo Red staining with other amyloid detection methods, highlighting when and why Congo Red remains the preferred choice in certain diagnostic and research scenarios.
Congo Red Stain vs. Other Amyloid Staining Techniques
The detection and characterization of amyloid deposits are critical in diagnosing various diseases, including amyloidosis and Alzheimer’s disease. While the Congo Red stain is the gold standard due to its unique apple-green birefringence under polarized light, several alternative staining techniques exist. This section compares Congo Red with other commonly used methods, highlighting their advantages, limitations, and ideal applications.
🏆 Congo Red Stain: The Gold Standard
- Key Feature: Produces apple-green birefringence under polarized light, specific for amyloid fibrils.
- Mechanism: Binds to the β-pleated sheet structure of amyloid, resulting in a distinctive optical property.
- Applications:
- Routine diagnosis of systemic and localized amyloidosis.
- Neuropathological studies (e.g., amyloid-β plaques in Alzheimer’s disease).
Strengths:
- High specificity for amyloid.
- Cost-effective and accessible.
- Rapid procedure with minimal equipment requirements.
Weaknesses:
- Requires expertise for birefringence interpretation.
- Cannot differentiate amyloid subtypes.
- Sensitivity issues with small or sparse deposits.
🧬 Thioflavin T and Thioflavin S Staining
✨ Key Features:
- Thioflavin T (ThT) and Thioflavin S (ThS) are fluorescent dyes that bind to amyloid fibrils.
- Under fluorescence microscopy, they emit green fluorescence, allowing sensitive detection.
🔬 Advantages:
- Higher sensitivity than Congo Red, detecting smaller amyloid deposits.
- Quantitative analysis possible with ThT fluorescence intensity measurement.
- Suitable for early-stage amyloid detection in research.
⚠️ Limitations:
- Less specific than Congo Red; other β-sheet-rich proteins may fluoresce.
- Requires fluorescence microscopy, which may not be available in all labs.
- Fluorescence intensity can fade over time, requiring immediate analysis.
🎯 Best Use:
- Research settings requiring high-sensitivity detection.
- In vitro studies of amyloid aggregation kinetics.
🏷️ Immunohistochemistry (IHC) for Amyloid Typing
🏥 Key Features:
- Uses antibodies specific to various amyloidogenic proteins (e.g., AL, AA, ATTR).
- Provides subtype identification, crucial for determining treatment strategies.
🌟 Advantages:
- Highly specific for amyloid subtypes.
- Enables differential diagnosis, which Congo Red cannot achieve.
- Permanent staining, allowing long-term slide storage.
❌ Limitations:
- Expensive, requiring specific antibodies and reagents.
- More technically complex than Congo Red staining.
- Longer processing times, delaying diagnostic turnaround.
🎯 Best Use:
- Clinical diagnostics requiring precise amyloid subtyping.
- Confirmation of systemic amyloidosis etiology.
🧪 Mass Spectrometry-Based Proteomic Analysis
🧫 Key Features:
- Provides definitive amyloid typing by analyzing the protein composition of amyloid deposits.
- Considered the most accurate method for identifying amyloid types.
💎 Advantages:
- Gold standard for amyloid subtyping.
- Can detect rare or atypical amyloid proteins.
- Extremely high specificity and sensitivity.
🏷️ Limitations:
- High cost and requires specialized equipment.
- Not widely available in routine pathology labs.
- Requires expertise in proteomics for data interpretation.
🎯 Best Use:
- Complex or ambiguous amyloidosis cases.
- When initial Congo Red and IHC results are inconclusive.
🔬 Electron Microscopy (EM)
🏛️ Key Features:
- Provides ultrastructural visualization of amyloid fibrils.
- Amyloid fibrils appear as non-branching, 7–10 nm filaments under EM.
🌐 Advantages:
- Definitive morphological confirmation of amyloid.
- Can detect early amyloid formation not visible with light microscopy.
🚫 Limitations:
- Highly technical and expensive.
- Not suitable for routine clinical use due to complexity.
- Time-consuming sample preparation.
🎯 Best Use:
- Research studies requiring detailed amyloid morphology.
- Confirmatory diagnosis in difficult clinical cases.
📊 Comparative Summary of Amyloid Staining Techniques
| Technique | Sensitivity | Specificity | Subtype Identification | Equipment Needed | Ideal Use |
|---|---|---|---|---|---|
| Congo Red | Moderate | High | No | Polarized light microscope | Routine amyloid detection |
| Thioflavin T/S | High | Moderate | No | Fluorescence microscope | Research; early-stage detection |
| Immunohistochemistry (IHC) | High | Very High | Yes | Antibodies, standard microscopy | Amyloid subtyping in diagnostics |
| Mass Spectrometry | Very High | Very High | Yes | Mass spectrometer | Definitive amyloid subtyping |
| Electron Microscopy (EM) | Very High | High | No | Electron microscope | Morphological research studies |
📝 Choosing the Right Technique: Key Considerations
- Clinical vs. Research Needs:
- Congo Red: Ideal for routine diagnostic purposes.
- Thioflavin T/S: Preferred in research for sensitive detection.
- Subtype Identification:
- Use IHC or mass spectrometry when subtype differentiation impacts treatment decisions.
- Resource Availability:
- Congo Red remains the best choice in resource-limited settings due to its low cost and simplicity.
- Techniques like EM and mass spectrometry are suited for specialized centers.
- Sensitivity Requirements:
- For detecting minimal amyloid deposits, Thioflavin T/S or mass spectrometry may offer superior performance.
📝 Conclusion
The Congo Red stain remains a fundamental tool in the detection and diagnosis of amyloid deposits, thanks to its high specificity and the characteristic apple-green birefringence under polarized light. Its widespread use in clinical and research settings highlights its reliability and cost-effectiveness. However, when greater sensitivity, subtype identification, or ultrastructural analysis is required, alternative techniques like Thioflavin staining, immunohistochemistry, mass spectrometry, and electron microscopy can complement Congo Red staining.
FAQs: Congo Red Stain
1. What is Congo Red stain used for?
Congo Red stain is primarily used for detecting amyloid deposits in tissues, which is crucial in diagnosing amyloidosis and Alzheimer’s disease. It binds to amyloid fibrils and exhibits distinctive apple-green birefringence under polarized light.
2. How does Congo Red stain work?
Congo Red works by binding to the β-pleated sheet structure of amyloid fibrils, a key feature of amyloid proteins. This binding causes Congo Red to emit apple-green birefringence under polarized light microscopy, helping to identify the presence of amyloid deposits.
3. Is Congo Red staining the most accurate method for amyloid detection?
Congo Red staining is one of the most widely used and cost-effective methods for amyloid detection, but it is not always the most sensitive or specific. For greater sensitivity and subtype identification, techniques like Thioflavin T staining or immunohistochemistry (IHC) may be more suitable in certain cases.
4. Can Congo Red stain be used for diagnosing Alzheimer’s disease?
Yes, Congo Red stain is commonly used in neuropathology to detect amyloid plaques, which are a hallmark of Alzheimer’s disease. However, it is typically used in conjunction with other clinical evaluations and diagnostic methods for a comprehensive diagnosis.
5. How do you interpret Congo Red stain results?
Interpretation of Congo Red stain results involves observing apple-green birefringence under polarized light. A positive result indicates the presence of amyloid deposits. The staining does not provide information about the amyloid subtype, which may require additional methods like IHC or mass spectrometry for confirmation.
6. What are the advantages of Congo Red stain over other amyloid staining techniques?
Congo Red staining is simple, cost-effective, and widely available. It is particularly effective for detecting amyloid deposits in routine diagnostic settings. However, its limitations include lower sensitivity for small or sparse amyloid deposits and an inability to differentiate between amyloid subtypes.
7. How long does the Congo Red staining procedure take?
The Congo Red staining procedure typically takes around 1-2 hours, depending on the tissue preparation and staining steps. The process involves tissue fixation, staining, and examination under polarized light microscopy for accurate results.
8. Is Congo Red staining only used for amyloid detection?
While Congo Red is mainly used for amyloid detection, it has also been used in histopathology to stain other extracellular deposits in certain tissues. However, its primary role remains the detection of amyloid deposits in diseases like amyloidosis, Alzheimer’s, and Parkinson’s disease.
9. Can Congo Red staining be performed on frozen tissue samples?
Yes, Congo Red staining can be performed on both paraffin-embedded and frozen tissue sections. However, frozen tissue samples may require careful handling to preserve the integrity of amyloid deposits and ensure reliable staining results.
10. Are there any limitations of Congo Red staining?
While Congo Red staining is widely used, its limitations include lower sensitivity for small or sparse amyloid deposits, difficulty in identifying amyloid subtypes, and the need for polarized light microscopy for result interpretation. In cases where subtype identification is critical, additional methods like IHC or mass spectrometry are recommended.