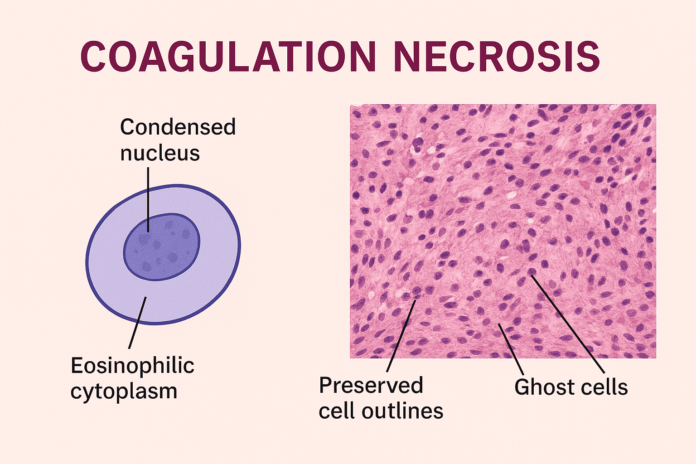
Coagulation necrosis is a distinct form of irreversible cell injury commonly observed in tissues affected by ischemia, such as the heart, kidneys, and spleen. Characterized by the preservation of tissue architecture despite cellular death, this type of necrosis plays a crucial role in various pathological conditions, including myocardial infarction.
In this blog post, we’ll explore the mechanisms behind coagulation necrosis, its histological features, clinical examples, and how it differs from other forms of necrosis like liquefactive and caseous necrosis.
2. What is Coagulation Necrosis?
Coagulation necrosis is a type of tissue death that occurs as a result of severe and irreversible cell injury, most commonly due to ischemia (lack of blood supply) or hypoxia (lack of oxygen). It is characterized by the preservation of the general tissue architecture, even though the individual cells are dead.
In this form of necrosis, the denaturation of cellular proteins—including enzymes—prevents the immediate breakdown of cellular structures. As a result, the cells lose their nuclei, but their outlines remain visible under a microscope for several days. This gives the tissue a ghost-like appearance.
The name “coagulation” refers to the coagulative or solidifying effect caused by protein denaturation, similar to how heat coagulates egg white. This type of necrosis is typical in solid organs such as the heart, kidneys, and spleen, where blood supply is suddenly cut off, leading to infarction.
Unlike liquefactive necrosis, which results in tissue dissolution, coagulation necrosis maintains tissue shape for a limited time, making it easier for pathologists to recognize the area of damage during histological examination.
3. Pathophysiology and Mechanism
The pathophysiology of coagulation necrosis involves a series of cellular and molecular events triggered by ischemic injury or toxic insults. The process begins when a tissue is deprived of oxygen and essential nutrients, leading to ATP depletion and loss of cellular homeostasis.
Key Steps in the Mechanism:
- ATP Depletion
The reduction in ATP levels impairs ion pumps (like Na⁺/K⁺-ATPase), causing an influx of sodium and water, leading to cellular swelling. - Calcium Influx
Disrupted calcium homeostasis activates intracellular enzymes (such as proteases, phospholipases, and endonucleases) that begin to degrade cellular components. - Protein Denaturation
One of the hallmarks of coagulative necrosis is the denaturation of structural proteins and enzymes, especially due to increased intracellular calcium and oxidative stress. This halts enzymatic digestion of the dead cells. - Preservation of Tissue Architecture
Despite cellular death, the basic outline of the cells and tissue remains intact for several days. This is because lysosomal enzymes are inactivated due to protein denaturation, delaying autolysis. - Inflammatory Response
Dead cells release damage-associated molecular patterns (DAMPs) that attract inflammatory cells, primarily neutrophils, which gradually remove necrotic debris.
💡 Why Architecture is Preserved
The preservation of the tissue structure in coagulation necrosis is due to the early inactivation of hydrolytic enzymes, preventing immediate cell lysis. Over time, immune cells infiltrate the area and begin phagocytosis of the necrotic cells.
This mechanism is especially prominent in myocardial infarction, where early microscopic examination reveals eosinophilic, anucleate cells arranged in their normal architecture, surrounded by inflammatory cells.
4. Histological Features
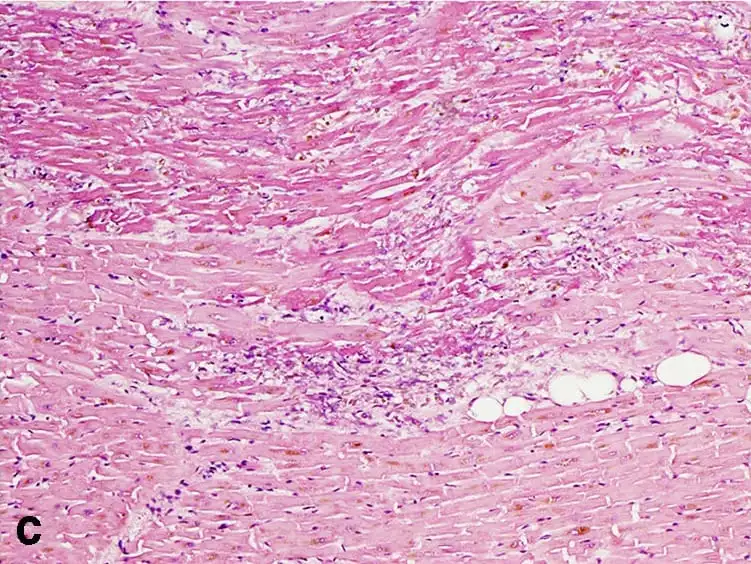
Under the microscope, coagulation necrosis presents a set of distinct histological characteristics that help differentiate it from other types of necrosis. Despite the death of the cells, the overall tissue architecture is preserved, at least in the early stages. This allows for easy identification of the affected area during histopathological examination.
🔍 Key Histological Characteristics:
- Eosinophilic Cytoplasm
The cytoplasm of necrotic cells becomes intensely pink (eosinophilic) due to protein denaturation and loss of basophilic RNA. - Loss of Nuclei
The nuclear material disappears, either through karyolysis (fading), pyknosis (shrinkage), or karyorrhexis (fragmentation), indicating irreversible cell death. - Cell Outlines Remain Intact
Despite nuclear loss, cell boundaries are still visible, giving the tissue a “ghost cell” appearance. This is due to the preservation of structural proteins that maintain cell shape. - Absence of Inflammatory Cells (Initially)
In the early stages, there may be minimal inflammatory infiltration, but as time progresses, neutrophils and macrophages begin to appear to clean up the necrotic debris. - Lack of Tissue Liquefaction
Unlike liquefactive necrosis, coagulative necrosis does not result in tissue softening or dissolution in the early phase. The tissue retains a firm consistency.
🧪 Common Sites for Observation:
- Heart: Following myocardial infarction, you’ll see coagulative necrosis within 12–24 hours of ischemic injury.
- Kidneys: Acute renal infarction shows sharply demarcated areas of necrotic tubules.
- Spleen and Adrenal Glands: Also common sites where coagulative necrosis can be observed due to infarction.
Histological staining with Hematoxylin and Eosin (H&E) is the gold standard for observing these features, especially in clinical and academic pathology.
5. Causes and Clinical Examples
Coagulation necrosis most commonly occurs as a result of ischemic injury, but it can also be triggered by toxins, infections, and certain chemical exposures. The hallmark is the sudden loss of oxygen and nutrients, leading to cell death while maintaining the structural integrity of the tissue—at least temporarily.
✅ Common Causes:
- Ischemia (Reduced Blood Flow)
- The most frequent cause of coagulative necrosis.
- Results from arterial blockage or hypoperfusion.
- Affects organs with end-arterial blood supply like the heart, kidneys, and spleen.
- Infarction
- Localized area of ischemic necrosis due to obstruction (e.g., thrombus or embolus).
- Seen in myocardial infarction, renal infarction, and splenic infarction.
- Chemical Injury
- Exposure to toxic substances (e.g., mercury, cyanide) can cause direct cell injury and coagulative necrosis.
- Thermal Injury
- Burns and heat exposure can denature proteins and result in coagulative changes.
- Certain Infections
- Some bacterial infections can induce coagulative necrosis as a component of the host’s immune response.
🏥 Clinical Examples:
- Myocardial Infarction (Heart Attack)
- One of the classical examples.
- Within 12–24 hours, cardiomyocytes show coagulative necrosis with preserved cell outlines and no nuclei.
- Renal Infarction
- Often secondary to emboli or thrombosis.
- Histology shows sharply defined areas of pale necrosis with intact tubule architecture.
- Splenic Infarction
- Can result from sickle cell disease or embolic events.
- Gross examination shows wedge-shaped pale areas; microscopically, coagulative necrosis is evident.
- Adrenal Gland Necrosis (Waterhouse-Friderichsen Syndrome)
- Caused by massive adrenal hemorrhage in septicemia, especially with Neisseria meningitidis.
- Testicular Torsion
- Twisting of the spermatic cord causes ischemia, resulting in coagulative necrosis of testicular tissue.
These clinical examples highlight the widespread occurrence and pathological relevance of coagulative necrosis in systemic diseases.
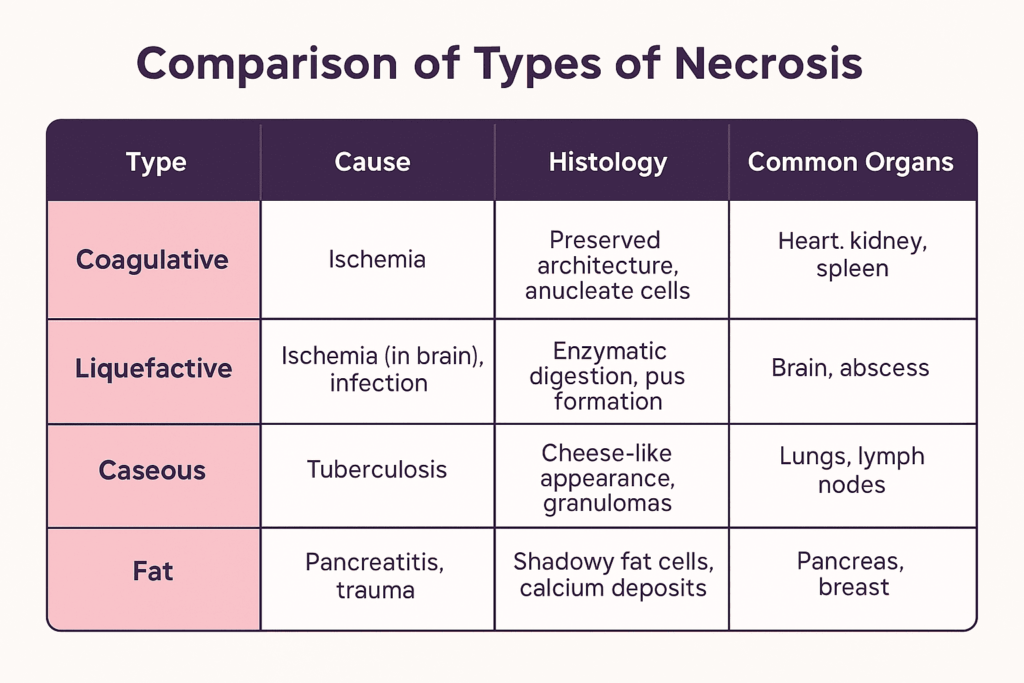
6. Coagulation Necrosis vs Other Types of Necrosis
While coagulation necrosis is one of the most common types of necrosis, it’s important to differentiate it from other forms that occur under different pathological conditions. Each type has distinct mechanisms, histological features, and clinical implications.
🧬 Coagulation Necrosis
- Cause: Ischemia or hypoxia (except in the brain)
- Tissue architecture: Preserved for several days
- Histology: Eosinophilic cytoplasm, anucleate cells, intact outlines
- Common organs: Heart, kidneys, spleen
- Example: Myocardial infarction
💧 Liquefactive Necrosis
- Cause: Ischemia in the brain, bacterial infections
- Tissue architecture: Destroyed; tissue becomes liquid
- Histology: Digested cells, pus formation, abundant neutrophils
- Common organs: Brain, abscesses in any tissue
- Example: Cerebral infarction, abscess due to Staphylococcus aureus
🧀 Caseous Necrosis
- Cause: Tuberculosis (TB), certain fungal infections
- Tissue architecture: Loss of structure; “cheesy” appearance
- Histology: Granulomatous inflammation, central necrosis
- Common organs: Lungs, lymph nodes
- Example: Pulmonary TB
🧫 Fat Necrosis
- Cause: Enzymatic digestion of fat by lipases (e.g., in pancreatitis)
- Tissue architecture: Disrupted fat cells, chalky deposits
- Histology: Fat saponification with calcium deposits
- Common organs: Pancreas, breast
- Example: Acute pancreatitis
⚡ Fibrinoid Necrosis
- Cause: Immune reactions involving blood vessels (vasculitis, hypertension)
- Tissue architecture: Not preserved
- Histology: Bright pink fibrin-like material in vessel walls
- Common sites: Blood vessels
- Example: Polyarteritis nodosa, malignant hypertension
🧯 Gangrenous Necrosis (Not a true type, but a clinical pattern)
- Dry gangrene: Coagulation necrosis of a limb (ischemic)
- Wet gangrene: Coagulation + liquefactive necrosis due to infection
🧾 Summary Table
| Type | Cause | Tissue Architecture | Key Features |
|---|---|---|---|
| Coagulation | Ischemia (except brain) | Preserved | Firm tissue, anucleate cells |
| Liquefactive | Brain infarct, abscess | Destroyed | Pus, digested tissue |
| Caseous | TB, fungal infection | Destroyed | Granulomas, cheesy necrosis |
| Fat | Pancreatitis, trauma | Variable | Chalky, saponified fat |
| Fibrinoid | Autoimmune, hypertension | Not preserved | Fibrin deposits in vessels |
| Gangrenous | Ischemia + infection | Varies | Dry (coagulative), Wet (mixed) |
7. Coagulation Necrosis vs Apoptosis
Although both coagulation necrosis and apoptosis involve cell death, they are fundamentally different in their mechanism, morphology, and biological consequences. Understanding these differences is crucial for distinguishing between pathological cell injury and programmed cellular processes.
🧬 Key Differences Between Coagulation Necrosis and Apoptosis
| Feature | Coagulation Necrosis | Apoptosis |
|---|---|---|
| Type of Cell Death | Accidental, uncontrolled | Programmed, regulated |
| Cause | Ischemia, toxins, trauma | Physiological signals, mild DNA damage |
| Energy Dependence | Passive (due to ATP depletion) | Active (requires ATP) |
| Cell Size | Swelling (oncosis) | Shrinkage |
| Membrane Integrity | Lost — leads to leakage of cell contents | Maintained — no leakage |
| Nuclear Changes | Karyolysis, pyknosis, or karyorrhexis | Nuclear condensation and fragmentation |
| Inflammation | Yes — triggers strong inflammatory response | No — apoptotic bodies are phagocytosed silently |
| Histological Features | Eosinophilic cytoplasm, preserved tissue structure | Cell shrinkage, apoptotic bodies, no tissue damage |
| Biological Impact | Always pathological | Can be physiological or pathological |
| Examples | Myocardial infarction, renal infarct | Embryonic development, immune cell regulation |
🔍 Visual Summary:
- Coagulation Necrosis:
– Massive cell death in a local tissue area
– Cells rupture and spill contents → inflammation
– Seen in infarcts of heart, kidney, spleen - Apoptosis:
– Individual cell death without harming neighbors
– Cell neatly dismantles itself into apoptotic bodies
– Common during normal tissue remodeling or immune regulation
🔑 Clinical Insight:
- Coagulation necrosis is typically associated with acute pathological conditions, such as myocardial infarction or ischemic injury.
- Apoptosis plays a critical role in normal development, immune function, and eliminating damaged cells without collateral damage.
10. FAQs About Coagulation Necrosis
❓ What is the main cause of coagulation necrosis?
The primary cause of coagulation necrosis is ischemia—a condition where blood flow (and thus oxygen) is restricted or reduced in a part of the body. This most commonly results from arterial blockage, such as in myocardial infarction (heart attack) or renal infarction. Other causes include chemical injury, thermal burns, and certain infections.
❓ Is coagulation necrosis reversible?
No, coagulation necrosis is irreversible. Once the cells are deprived of oxygen and nutrients long enough to undergo necrosis, the damage becomes permanent. However, early intervention in ischemic conditions may prevent progression to necrosis if blood flow is quickly restored.
❓ What organs are commonly affected?
Coagulation necrosis typically affects solid organs with a limited collateral blood supply, including:
- Heart – after a heart attack (myocardial infarction)
- Kidneys – following a renal artery blockage
- Spleen – due to infarction
- Adrenal glands and testes – in cases like adrenal hemorrhage or testicular torsion
❓ How is coagulation necrosis diagnosed?
Diagnosis involves a combination of clinical evaluation, imaging, and histopathological analysis:
- Imaging (e.g., MRI, CT scan) may show non-perfused areas in ischemic tissues.
- Biopsy and histology (H&E stain) reveal hallmark features such as:
- Eosinophilic cytoplasm
- Preserved cell outlines
- Loss of nuclei
- In cardiac events, blood markers like troponins also support the diagnosis.
Conclusion
Coagulation necrosis is a classic pattern of tissue injury, most often resulting from ischemia. Its distinct histological features and underlying mechanisms make it a key concept in pathology and clinical diagnosis. By understanding its causes, appearance, and how it differs from other forms of necrosis and apoptosis, healthcare professionals and students can better interpret tissue changes and disease processes.