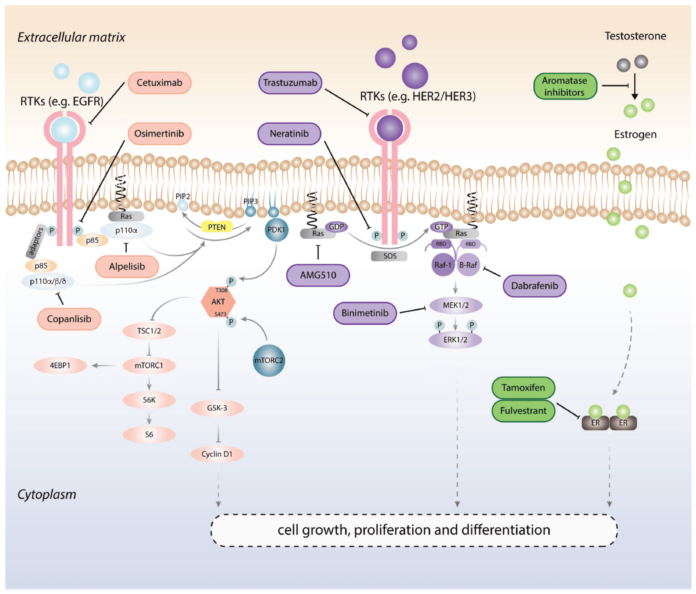
Cell signaling is the foundation of cellular communication, enabling cells to respond to internal and external cues. This complex process governs critical cellular functions such as growth, differentiation, survival, and apoptosis. Through a network of signaling molecules, receptors, and pathways, cells maintain homeostasis and coordinate their activities within tissues and organs.
However, in cancer, these signaling pathways often become dysregulated. Genetic mutations, epigenetic changes, or aberrant protein activity can hijack normal signaling processes, driving uncontrolled cell growth, evasion of apoptosis, and metastasis.
This article explores the major cell signaling pathways involved in cancer, their roles in tumor progression, and the current therapeutic approaches targeting these pathways.
Overview of Cell Signaling Pathways in Cancer
Cell signaling pathways are essential for maintaining cellular functions and ensuring the body’s proper physiological balance. These pathways are networks that transmit signals from the cell surface to the nucleus, orchestrating a variety of cellular processes. In the context of cancer, these pathways often become dysregulated, fueling the development and progression of tumors.
Key Functions of Cell Signaling:
- Regulation of Cell Proliferation and Differentiation:
Normal signaling pathways regulate when cells should divide or differentiate into specific cell types. For example, growth factors like epidermal growth factor (EGF) bind to their receptors, triggering cascades such as the MAPK/ERK pathway to promote controlled cell division. Dysregulation can lead to excessive proliferation, a hallmark of cancer. - Apoptosis and Cell Survival Mechanisms:
Cell signaling determines whether a cell should survive or undergo programmed cell death (apoptosis). For instance, the PI3K/AKT pathway promotes survival by inhibiting pro-apoptotic factors. In cancer, mutations often upregulate survival signals, allowing malignant cells to evade apoptosis and persist despite damage. - Communication with the Tumor Microenvironment:
Cancer cells exploit signaling to interact with their surroundings, recruiting stromal cells, immune cells, and blood vessels to support tumor growth. Pathways like VEGF signaling promote angiogenesis, ensuring the tumor receives sufficient nutrients and oxygen.
Impact of Dysregulated Signaling:
- Oncogenic Mutations:
Mutations in key signaling proteins can transform normal cells into cancerous ones. For example, mutations in KRAS or BRAF genes result in the constant activation of proliferative pathways, even in the absence of external signals. - Pathway Activation in Tumor Growth and Metastasis:
Aberrant activation of pathways like Wnt/β-catenin or Hedgehog signaling supports tumor growth and metastasis. These pathways can enhance cell motility, invasion, and colonization of distant organs.
Major Oncogenic Signaling Pathways
Oncogenic signaling pathways are central to the transformation of normal cells into cancer cells. These pathways regulate critical processes like cell proliferation, survival, and metabolism. In cancer, mutations or overactivation of these pathways drive tumor progression and therapy resistance. Below are some of the most prominent pathways implicated in oncogenesis.
MAPK/ERK Pathway
The MAPK/ERK pathway is a critical regulator of cell proliferation, differentiation, and survival. It is activated by growth factors binding to receptor tyrosine kinases (RTKs), which trigger a cascade involving RAS, RAF, MEK, and ERK proteins.
- Role in Cell Proliferation and Survival:
The MAPK/ERK pathway promotes the expression of genes involved in cell division and survival, making it a key driver of tumor growth. - Mutations in KRAS and BRAF in Cancer:
Mutations in KRAS and BRAF genes are common in cancers such as lung, colorectal, and melanoma. These mutations lead to constant pathway activation, driving uncontrolled cell proliferation even without external signals.
PI3K/AKT/mTOR Pathway
The PI3K/AKT/mTOR pathway regulates cellular metabolism, growth, and survival, making it a central player in tumorigenesis.
- Mechanisms in Metabolism and Tumor Progression:
This pathway enhances glucose uptake, lipid synthesis, and protein translation to meet the metabolic demands of rapidly dividing cancer cells. - Therapeutic Inhibitors and Current Research:
Drugs like Everolimus (mTOR inhibitor) and PI3K inhibitors are being explored to target this pathway. However, resistance mechanisms often necessitate combination therapies.
Wnt/β-Catenin Pathway
The Wnt/β-catenin pathway is crucial for embryonic development and tissue regeneration. In cancer, its dysregulation plays a significant role in maintaining cancer stem cells and promoting metastasis.
- Importance in Cancer Stem Cell Maintenance:
Aberrant Wnt signaling helps cancer stem cells sustain self-renewal and resist therapies, contributing to tumor relapse. - Crosstalk with Other Pathways in Tumor Invasion:
Wnt signaling interacts with pathways like TGF-β and Hedgehog, enhancing tumor cell migration and invasion into surrounding tissues.
JAK/STAT Pathway
The JAK/STAT pathway is a key mediator of cytokine signaling, regulating immune responses and cell survival. Its dysregulation in cancer contributes to both tumor growth and immune evasion.
- Role in Inflammation and Immune Evasion:
Persistent activation of JAK/STAT signaling promotes an inflammatory tumor microenvironment and suppresses anti-tumor immunity. This allows cancer cells to evade detection and destruction by the immune system.
Role of Tumor Microenvironment in Cell Signaling
The tumor microenvironment (TME) plays a crucial role in cancer progression. Through complex signaling interactions, the TME supports tumor growth, metastasis, and resistance to therapies.
Interplay Between Tumor and Stroma
The tumor and surrounding stromal cells engage in bidirectional communication through signaling pathways, promoting tumor survival and progression.
- Cancer-Associated Fibroblasts and Signaling Activation:
Cancer-associated fibroblasts (CAFs) are reprogrammed stromal cells that secrete growth factors, cytokines, and extracellular matrix components. These molecules activate pathways like TGF-β and Wnt/β-catenin, supporting tumor invasion and metastasis. - Immune Cells and Cytokine Signaling in the Tumor Microenvironment:
Immune cells within the TME, such as macrophages and T-cells, often adopt tumor-promoting roles. For example, tumor-associated macrophages (TAMs) release cytokines like IL-6 and IL-10, which activate the JAK/STAT pathway to suppress anti-tumor immunity and enhance cancer cell survival.
Angiogenesis Pathways
Angiogenesis is a hallmark of cancer that ensures the tumor receives sufficient oxygen and nutrients for growth.
- VEGF Signaling in Tumor Vascularization:
Vascular endothelial growth factor (VEGF) is a key driver of angiogenesis. It binds to VEGF receptors on endothelial cells, activating pathways like PI3K/AKT and MAPK/ERK to stimulate blood vessel formation. Tumor cells often overexpress VEGF, creating an abnormal vascular network that facilitates tumor growth and metastasis. - Therapeutic Targeting of Angiogenesis:
Anti-angiogenic therapies, such as Bevacizumab (a VEGF inhibitor), aim to disrupt tumor vascularization. These treatments can normalize blood vessels, improving drug delivery and reducing tumor growth.
Crosstalk Among Pathways
Cancer progression is rarely driven by a single pathway; instead, multiple signaling pathways work in concert within the TME.
- How Multiple Pathways Contribute to Cancer Progression:
Crosstalk between pathways like PI3K/AKT, Wnt/β-catenin, and Notch amplifies tumor-promoting signals. For instance, the activation of Wnt signaling in cancer stem cells can enhance angiogenesis via VEGF, while TGF-β signaling modulates immune suppression. This interconnected signaling network creates a resilient tumor ecosystem that is challenging to target with single-agent therapies.
Therapeutic Targeting of Cell Signaling Pathways
Targeting dysregulated cell signaling pathways has revolutionized cancer therapy, offering more precise and effective treatment options. These therapies aim to interrupt the aberrant signals that drive tumor growth, survival, and metastasis.
Despite significant progress, challenges like drug resistance and pathway redundancy remain obstacles, underscoring the need for innovative approaches.
Targeted Therapy Approaches
- Tyrosine Kinase Inhibitors (TKIs):
Tyrosine kinases play a pivotal role in transmitting growth and survival signals within cells. TKIs, such as Erlotinib and Gefitinib, inhibit receptors like EGFR, effectively halting uncontrolled cell proliferation in cancers such as non-small cell lung cancer. - Monoclonal Antibodies:
Monoclonal antibodies target specific proteins on cancer cells. For example, Trastuzumab (Herceptin) binds to the HER2 receptor, blocking its activation in HER2-positive breast cancer. This approach not only disrupts signaling but also recruits immune cells to attack tumor cells.
Emerging Therapies
- mTOR Inhibitors and Their Potential:
The mTOR pathway regulates cell growth and metabolism, and its dysregulation is implicated in many cancers. Drugs like Everolimus and Temsirolimus inhibit mTOR, showing promise in renal cell carcinoma and hormone receptor-positive breast cancer. - Role of microRNAs in Modulating Signaling:
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression, including key signaling pathways. Therapeutic strategies involving miRNAs, such as miRNA mimics or inhibitors, are being developed to restore normal signaling and suppress tumor growth. For example, miR-34 mimics target the p53 pathway, enhancing apoptosis in cancer cells.
Challenges in Treatment Targeting Cell Signaling
- Drug Resistance Mechanisms:
Tumors often develop resistance to targeted therapies through secondary mutations, pathway redundancy, or compensatory activation of alternative pathways. For instance, mutations in the EGFR gene can render TKIs ineffective, necessitating the development of next-generation inhibitors. - Need for Combination Therapies:
Monotherapies targeting a single pathway are often insufficient due to the complex nature of cancer signaling networks. Combination therapies, which simultaneously target multiple pathways or incorporate immunotherapy, are emerging as a strategy to overcome resistance and improve outcomes. For example, combining VEGF inhibitors with PD-1/PD-L1 checkpoint inhibitors has shown enhanced efficacy in renal and lung cancers.
Future Directions and Research in Cancer Cell Signaling
The future of cancer therapy lies in unraveling the complexities of signaling pathways and leveraging this knowledge to enhance diagnosis, prognosis, and treatment. Advances in research are opening new avenues for precision medicine, innovative therapeutic strategies, and more effective tools for monitoring disease progression.
Biomarkers for Diagnosis and Prognosis
- Role of Pathway-Specific Mutations (e.g., KRAS, BRAF):
Mutations in key signaling proteins serve as crucial biomarkers in cancer. For instance, KRAS mutations are common in colorectal and pancreatic cancers, while BRAF mutations, particularly V600E, are prevalent in melanoma. Detecting these mutations enables early diagnosis and stratification of patients for targeted therapies. - Importance of Personalized Medicine:
Personalized medicine tailors treatment based on a patient’s unique genetic and molecular profile. By identifying pathway-specific biomarkers, clinicians can design therapies that are more effective and have fewer side effects. For example, HER2 testing in breast cancer determines eligibility for Trastuzumab treatment.
Advances in Cell Signaling Research
- 3D Cell Culture Models for Pathway Analysis:
Traditional 2D cell cultures often fail to replicate the complexity of in vivo tumors. 3D cell culture models, such as organoids, provide a more accurate representation of tumor architecture and signaling dynamics. These models are invaluable for studying pathway interactions and testing potential inhibitors. - High-Throughput Screening for Pathway Inhibitors:
High-throughput screening technologies enable rapid testing of thousands of compounds to identify pathway-specific inhibitors. This approach has led to the discovery of promising drug candidates targeting pathways like PI3K/AKT and Wnt/β-catenin, accelerating the development of targeted therapies.
Potential of Multi-Targeted Therapies
- Combination Approaches Targeting Multiple Pathways:
Cancer is driven by a network of interconnected pathways, making single-target therapies often insufficient. Multi-targeted therapies aim to simultaneously inhibit several pathways to counteract resistance and achieve better outcomes. For instance, combining MEK inhibitors (targeting MAPK/ERK) with PI3K inhibitors has shown synergistic effects in preclinical models.
Conclusion
Understanding cell signaling pathways is crucial for unraveling cancer biology and developing effective therapies. While advancements in targeted treatments have revolutionized care, challenges like drug resistance and pathway redundancy persist. Continued research is essential to overcome these obstacles and improve outcomes, offering hope for more precise and durable cancer treatments.